Merus advances the world's first LGR5-targeted bispecific antibody into Phase 3 clinical trials
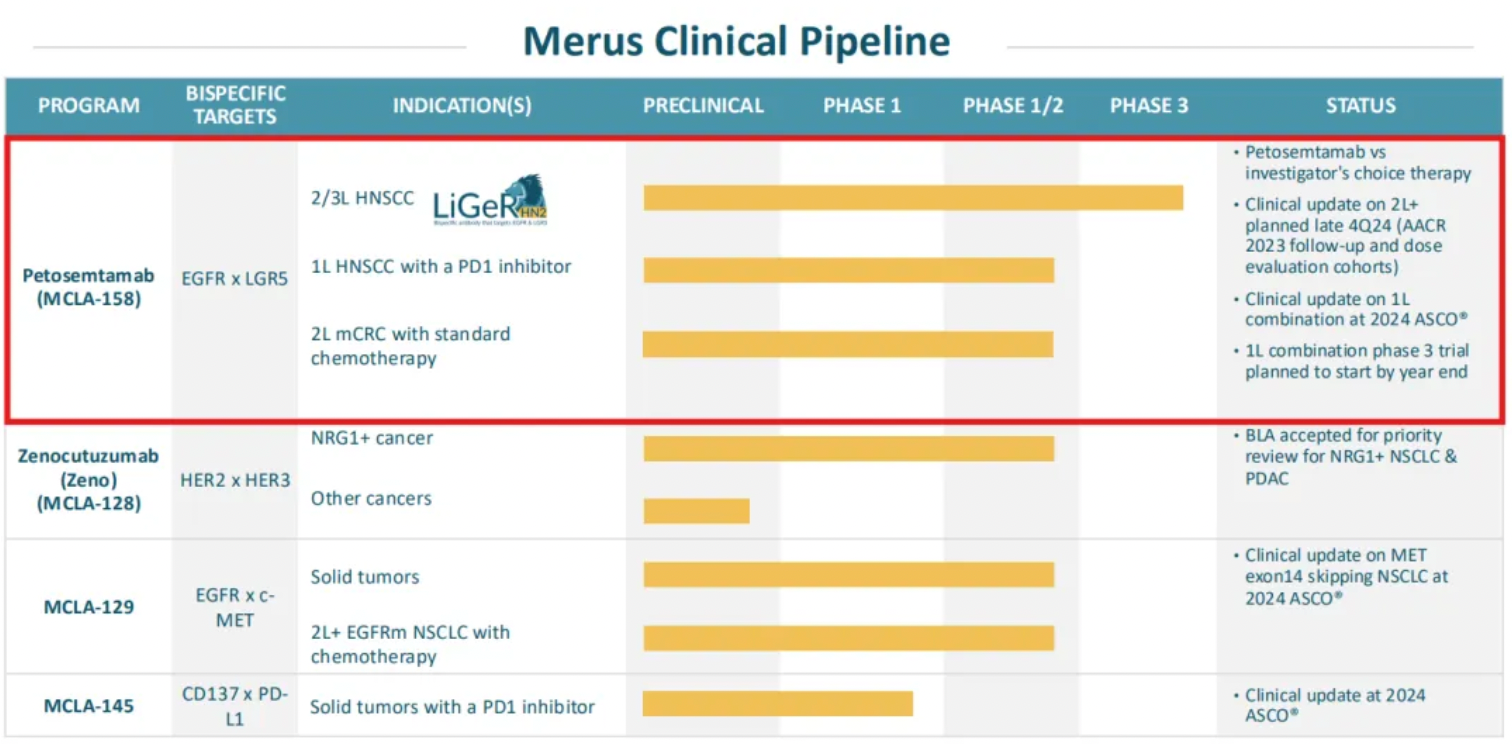
Recently, Merus announced that the first patient has been dosed in the phase 3 clinical trial LiGeR-HN2, aimed at evaluating the efficacy of petosemtamab in the second-line and third-line treatment of patients with recurrent or metastatic head and neck squamous cell carcinoma (r/m HNSCC).

LiGeR-HN2 is a randomized, open-label phase 3 clinical trial primarily assessing the safety and efficacy of petosemtamab compared to standard therapy in patients with recurrent or metastatic head and neck squamous cell carcinoma. The trial will enroll patients who have previously received first-line treatment and whose disease has progressed. The primary endpoints of the study typically include objective response rate (ORR), progression-free survival (PFS), overall survival (OS), and safety assessments.
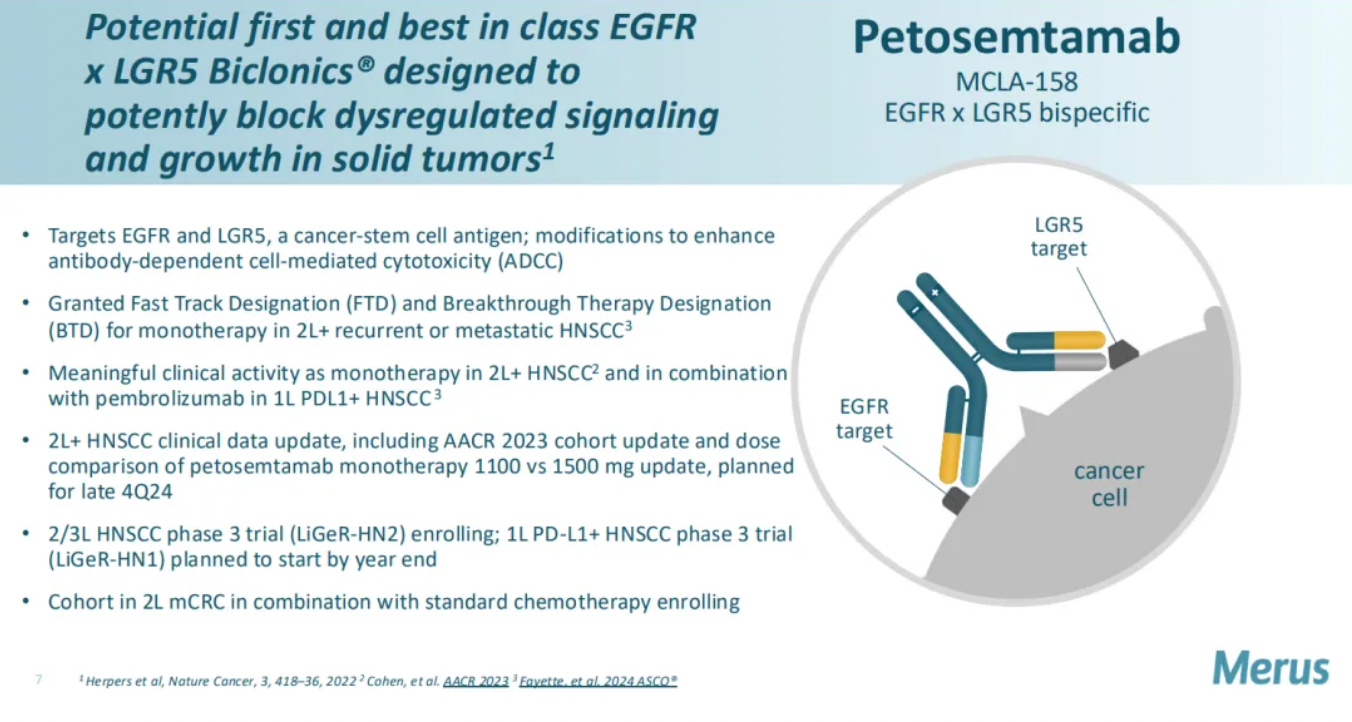
Petosemtamab is a bispecific antibody developed by Merus that simultaneously targets both the epidermal growth factor receptor (EGFR) and LGR5 (Leucine-rich repeat-containing G protein-coupled receptor 5), receptors which are overexpressed in various types of cancer. By targeting these two receptors, petosemtamab aims to more effectively target and kill cancer cells while reducing the impact on healthy cells.
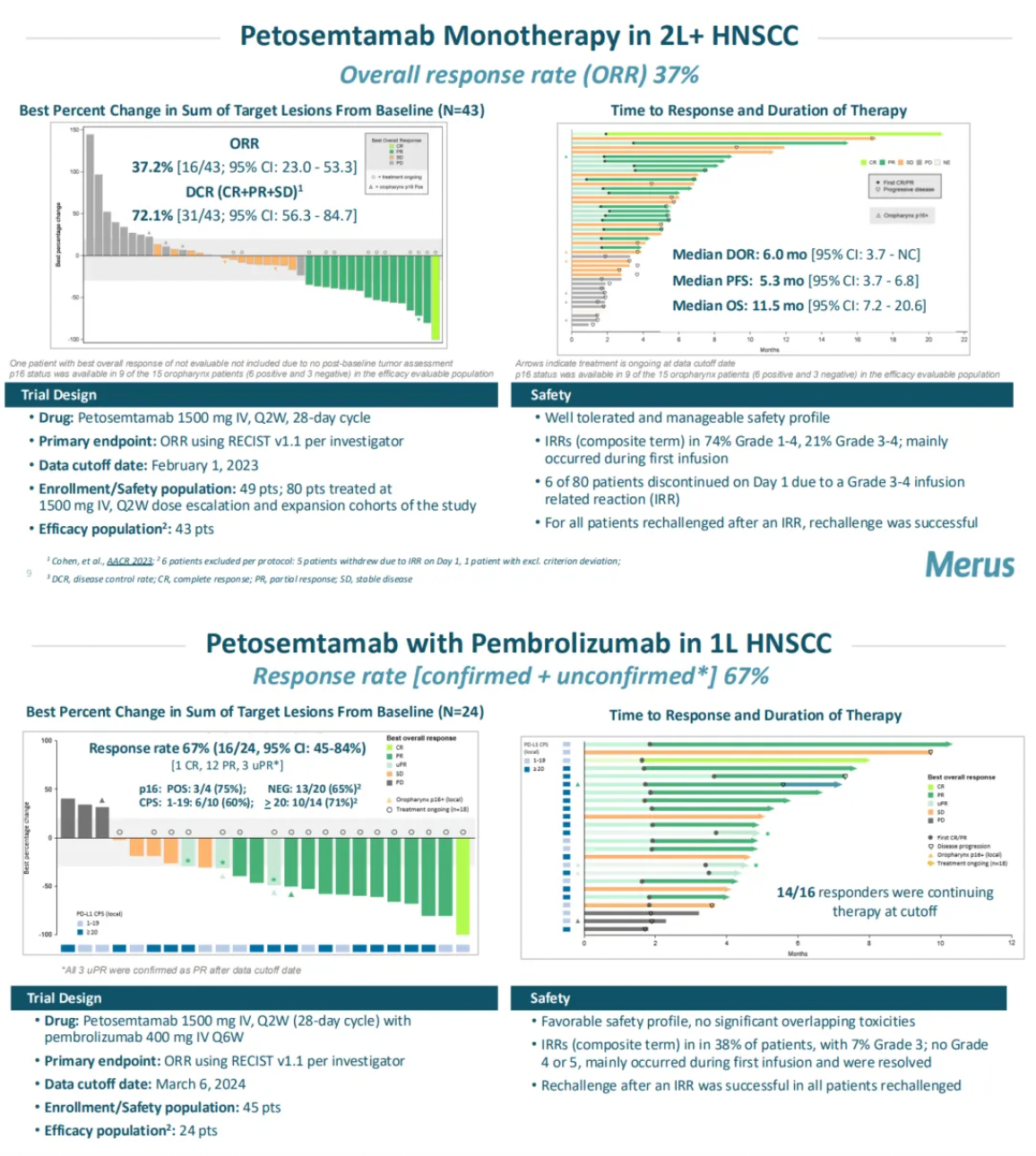
Data presented by Merus at the 2024 American Society of Clinical Oncology (ASCO) showed that in patients with head and neck squamous cell carcinoma (HNSCC), when used in combination with Keytruda, six out of ten evaluable patients demonstrated a tumor response, achieving a response rate of 60%.
Furthermore, Merus' latest public materials on their website also released mid-stage clinical updates of petosemtamab as a monotherapy for second-line and beyond HNSCC, which showed a response rate of 37% in 43 evaluable patients (2L+ HNSCC, ORR 37%), and mid-stage clinical updates of its combination with the PD-1 monoclonal antibody Keytruda for first-line HNSCC, which achieved a response rate of 67% in 24 evaluable patients (1L HNSCC, RR 67%).
The company projects that by 2028, the global market size for HNSCC will exceed $5 billion, highlighting the substantial commercial potential of petosemtamab for this indication.
The FDA has granted petosemtamab Breakthrough Therapy Designation (BTD) for the treatment of patients with recurrent or metastatic HNSCC who have experienced disease progression following platinum-based chemotherapy and anti-programmed cell death receptor-1 (PD-1) or anti-programmed death-ligand 1 (PD-L1) antibody therapy.
Partnering with Gilead
In early 2024, Gilead Sciences announced that it will pay Merus a $56 million upfront payment and make a $25 million equity investment in the biotech company.
Through the partnership with Merus, Gilead Sciences aims to leverage Merus's expertise in the development of bispecific and multispecific antibodies to create innovative antibody therapies. The specific targets of the collaboration have not been disclosed.
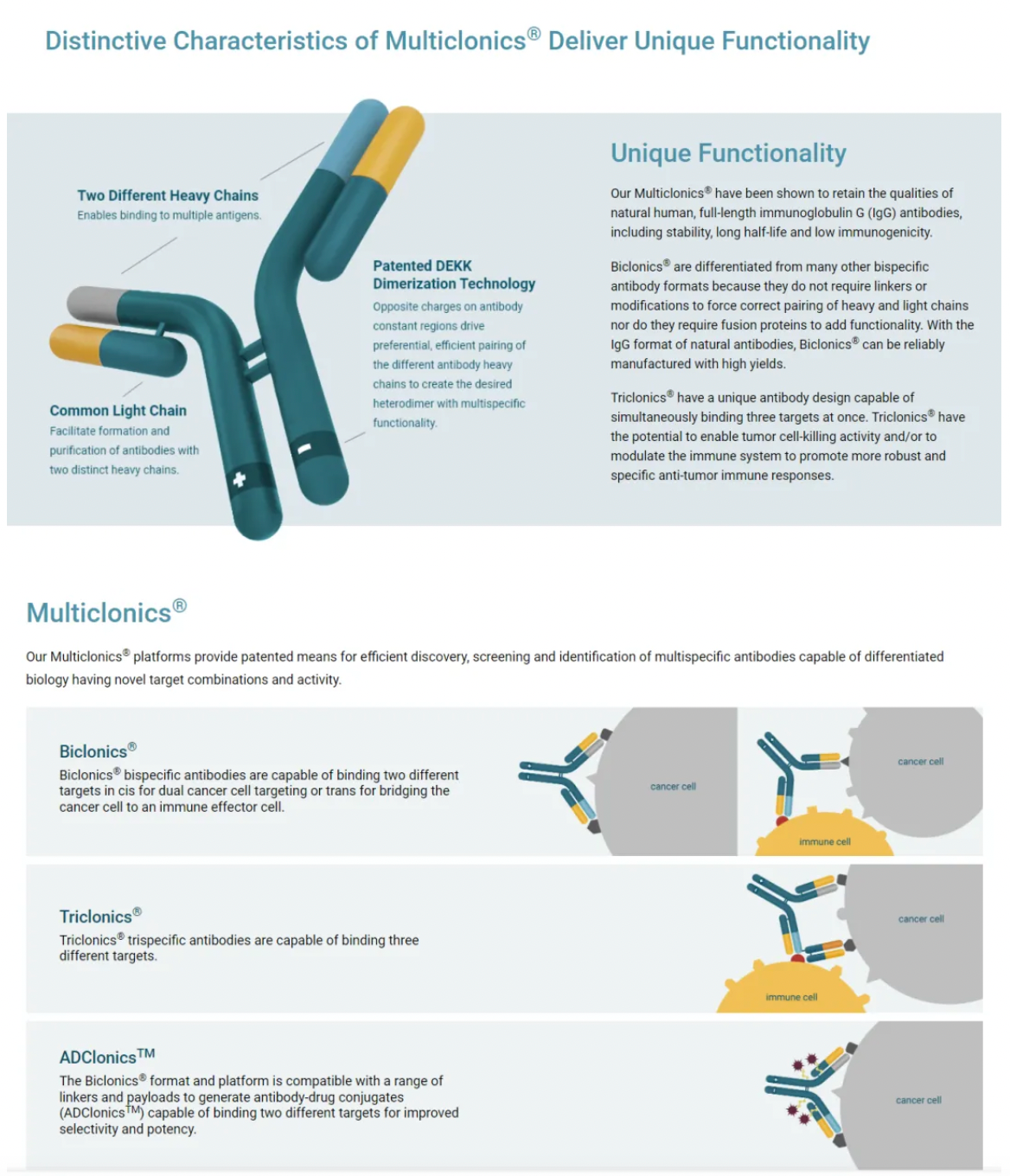
About LGR5
LGR5, also known as GPR49, is a seven-transmembrane protein that belongs to the GPCR superfamily. Within this GPCR superfamily, LGR5, along with LGR4 and LGR6 which are part of the glycoprotein-receptor orphan subgroup, share a distinctive structural feature: a large extracellular domain containing 17 leucine-rich repeat sequences. When LGR5 binds to its ligand R-Spondin (RSPO), it collaborates with the Wnt receptors (Frizzled and LRP5/6) to enhance the Wnt/β-catenin signaling pathway.
LGR5 regulates the Wnt/β-catenin signaling pathway in normal intestinal stem cells. For instance, during normal homeostasis of the intestine, the expression of LGR5 is restricted to the stem cell compartments located at the base of the crypts. As the progeny of the stem cells migrate upward through the proliferative zone and undergo differentiation, this expression of LGR5 is lost. Consistently, isolated single LGR5+ cells from the intestine can form self-organizing crypt/villus structures known as organoids, capable of reconstituting all differentiated epithelial lineages present in the intestine.
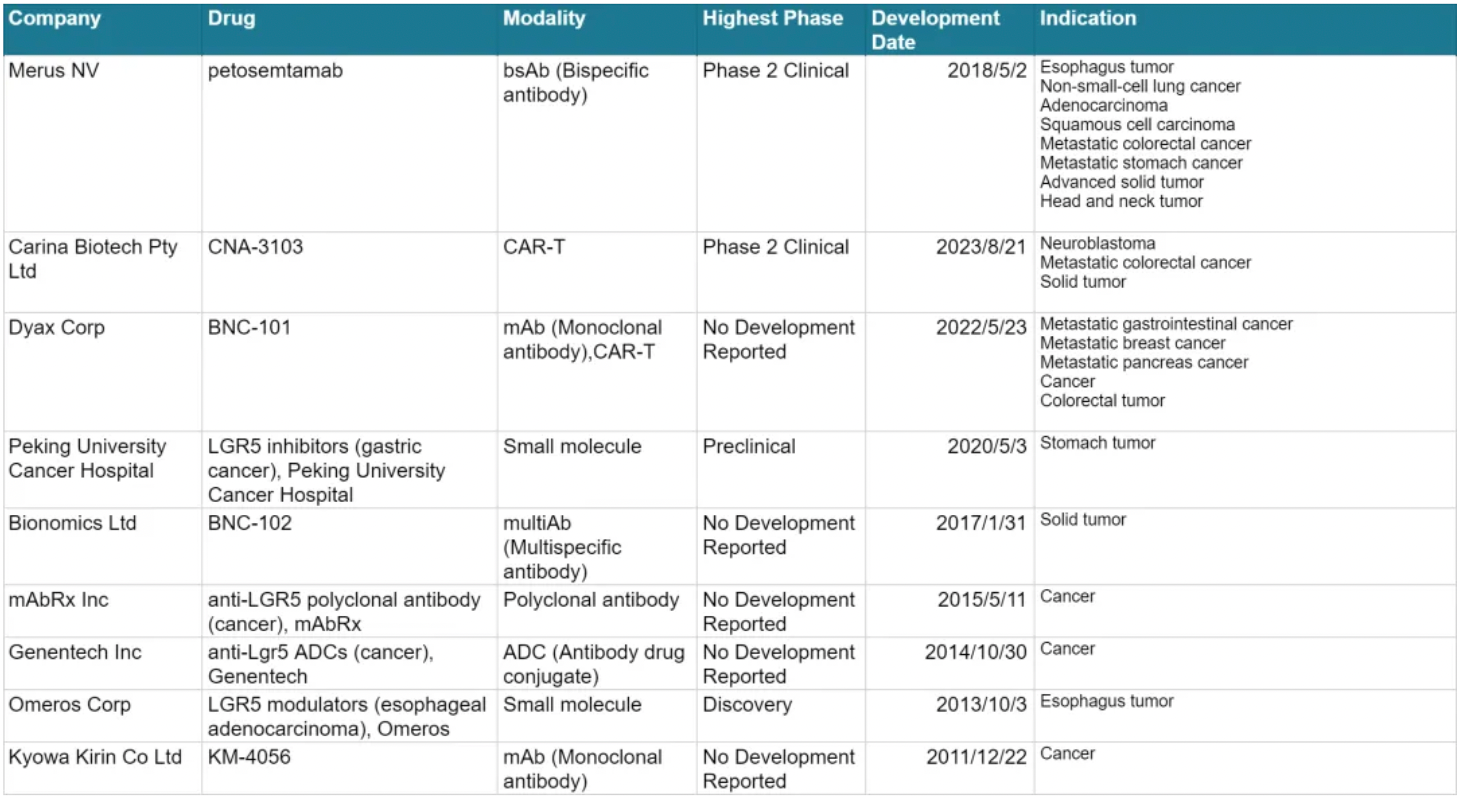
The table below presents the pipeline status of clinical studies related to LGR5, all of which are for cancer-related indications. Merus clearly holds a leading position in this target competition.
Next Indication: Colorectal Cancer
In an interview with the CEO of Merus, it was also mentioned that the company is looking forward to evaluating petosemtamab in metastatic colorectal cancer (mCRC), which represents another significant potential opportunity.
Colorectal cancer (CRC) is the third most commonly diagnosed malignancy worldwide and the fourth leading cause of cancer-related deaths, with its burden expected to increase by 60% by 2030. Despite significant improvements in detection and treatment protocols, most advanced-stage cancer patients will die from recurrence of their disease. A widely recognized reason for CRC recurrence is the failure of existing therapies to eradicate subpopulations of cancer stem cells (CSCs) within the tumor.
CSCs express a variety of markers, including CD133, CD44, CD166, ALDH, EphB2, and Leucine-rich repeat-containing G-protein coupled receptor 5 (LGR5). These CSCs are capable of resisting therapeutic assaults and re-establishing tumor growth after treatment interventions, creating an urgent need for innovative, non-toxic targeted cancer therapies to induce sustained clinical remission.
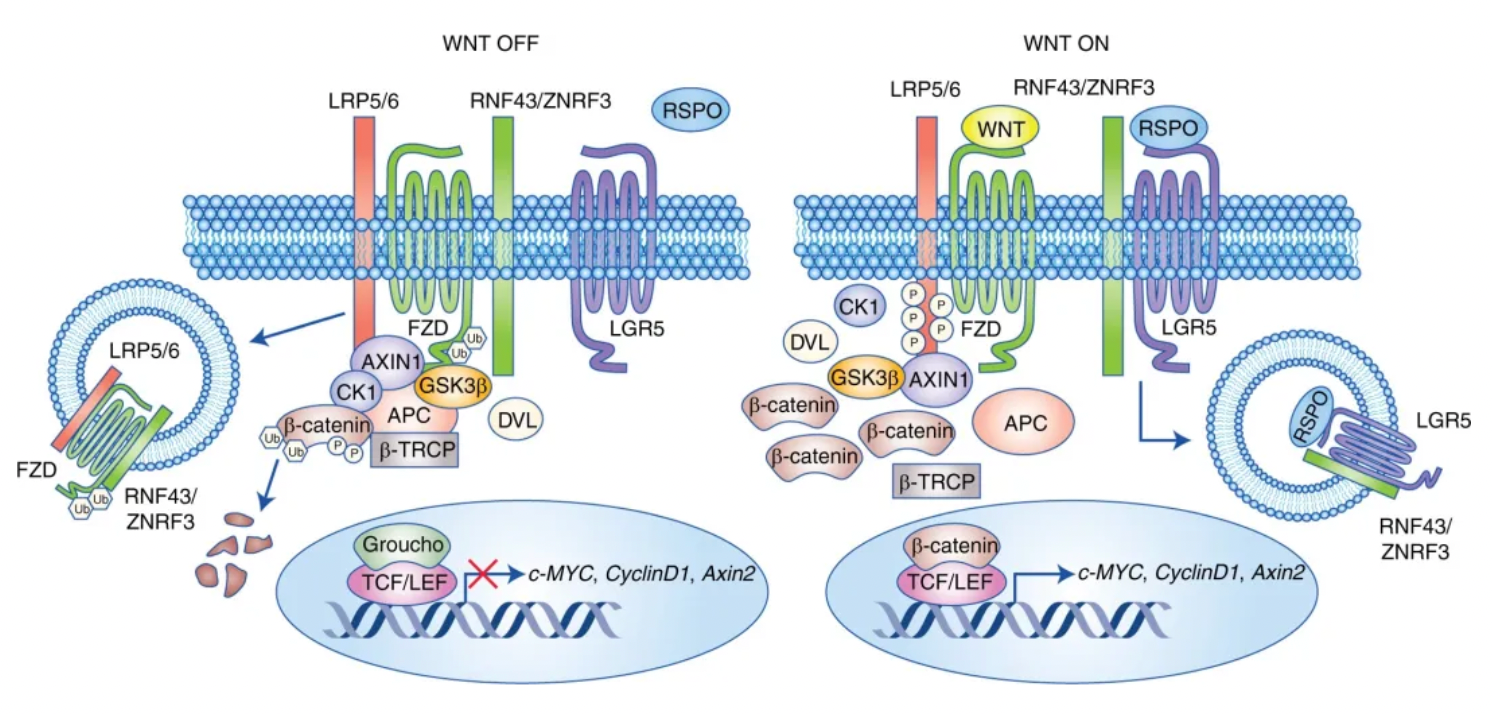
A key early event in the progression of colorectal cancer is the dysregulation of the Wnt/β-catenin signaling pathway, which is often constitutively activated by genetic mutations in APC or β-catenin. Under normal conditions, the lack of Wnt ligands leads to the constitutive phosphorylation of β-catenin, followed by proteasomal degradation, keeping Wnt target genes suppressed. When Wnt ligands bind to the Wnt receptors Frizzled and LRP5/6, the accumulation of β-catenin in the cytoplasm leads to its nuclear translocation, where it binds with the TCF/LEF family of transcription factors and activates oncogenic Wnt target genes such as c-myc, cyclinD1, and survivin.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!