Laninolanor - Small Molecule Drug Targeting PPARα/PPARγ/PPARδ
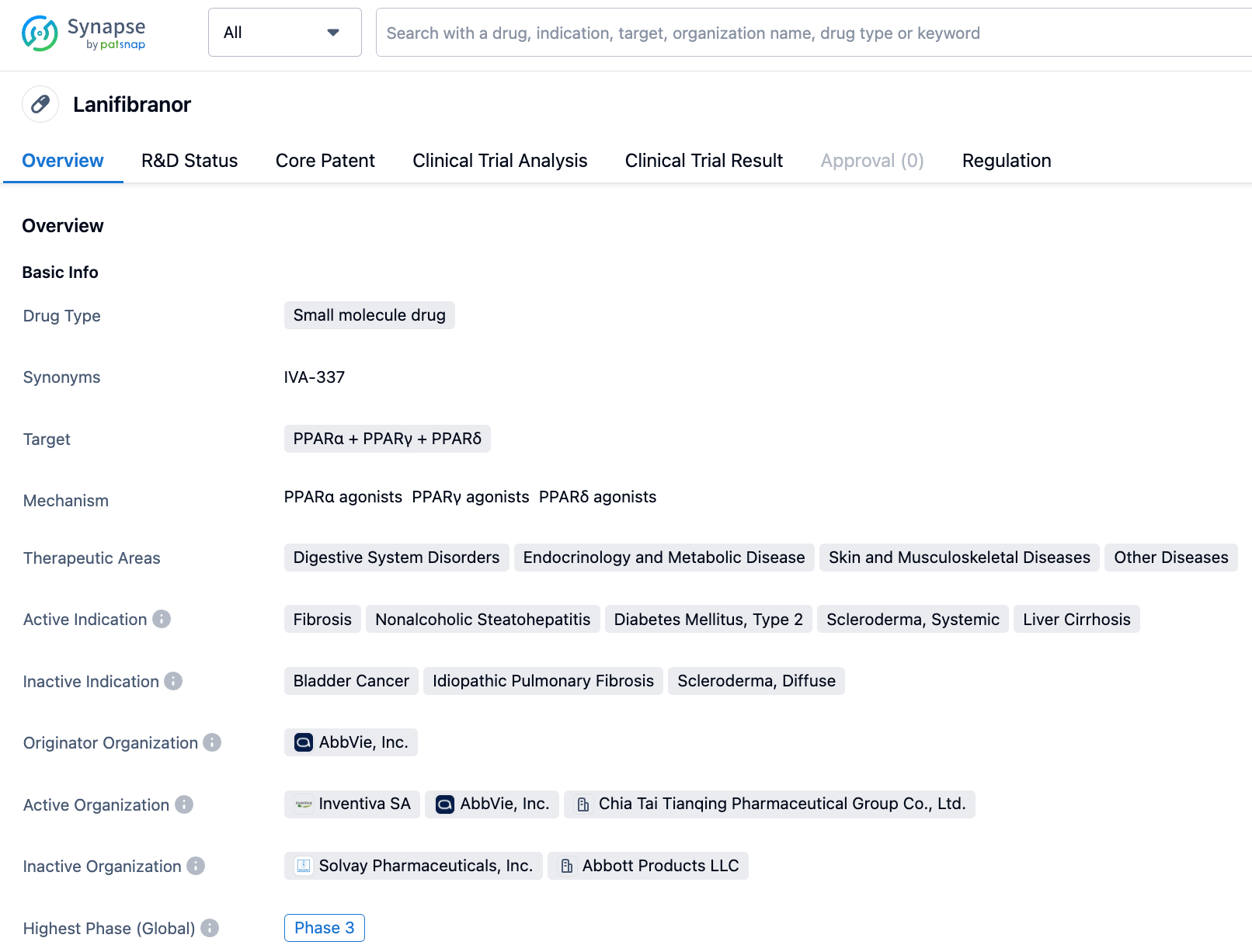
Lanifibranor, a small-molecule drug targeting PPARα + PPARγ + PPARδ, was initially developed by AbbVie, Inc. On September 21, 2022, Inventiva announced that it had signed a license and cooperation agreement with CStone Pharmaceuticals for the development and commercialization of the pan-PPAR agonist, lanifibranor, for the treatment of non-alcoholic steatohepatitis (NASH) and potential other metabolic diseases for the Chinese population. In July 2023, the drug was concurrently granted Breakthrough Therapy, Orphan Drug, and Rare Pediatric Disease designations by the FDA.
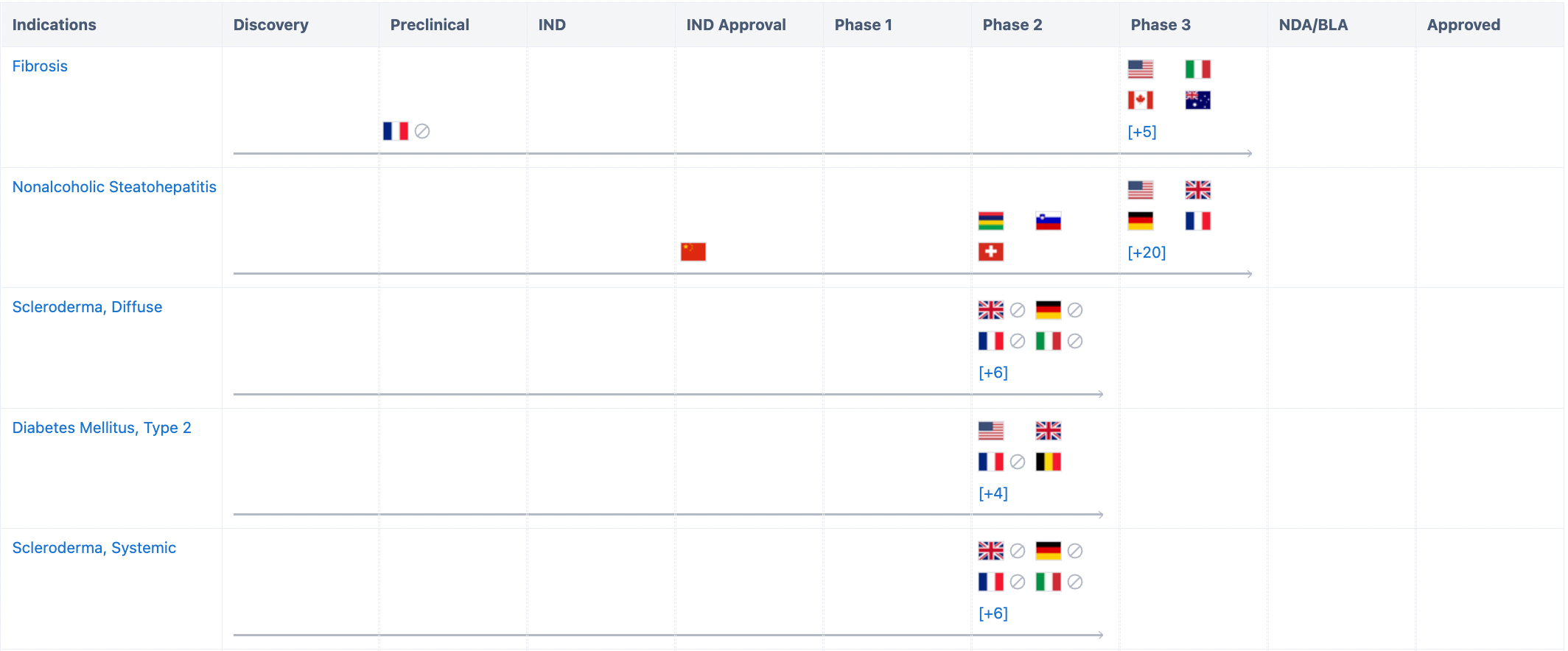
According to the "Synapse" database, the currently researched indications for this drug include fibrosis, non-alcoholic steatohepatitis, type 2 diabetes, systemic sclerosis, cirrhosis, and more. Among them, fibrosis has already entered Phase 3 clinical trials in multiple countries including the US, Italy, and Canada. For more detailed information.
This drug has recently been recognized as a rare breakthrough therapy for NASH. Moreover, on July 24, the NMPA plans to grant Lanifibranor breakthrough therapy certification.
Mechanism of Action
Lanifibranor can activate all three subtypes of PPAR: α, γ, δ. Preliminary studies suggest that lanifibranor can improve insulin sensitivity and macrophage activation, reduce liver fibrosis and inflammation gene expression. Its therapeutic effects exceed those of single or dual PPAR agonists.
Core Clinical Trials
In a phase 2b study (NCT03008070), the efficacy and safety of Lanifibranor for patients with non-alcoholic steatohepatitis were assessed. The trial included 247 participants, randomly assigned to the Lanifibranor 1200 mg group, the Lanifibranor 800 mg group, and the placebo group. The results showed that the fibrosis regression rate was 48% in the Lanifibranor 1200 mg group, 34% in the 800 mg group, and 29% in the placebo group. Furthermore, the NASH resolution rate was 49% in the Lanifibranor 1200 mg group, 39% in the 800 mg group, and 22% in the placebo group.
Competitive Landscape
Statistics show that there are currently eight pipeline drugs involving PPARα + PPARγ + PPARδ. Apart from Lanifibranor, no other Pan-PPAR drugs have made progress or terminated research and development.