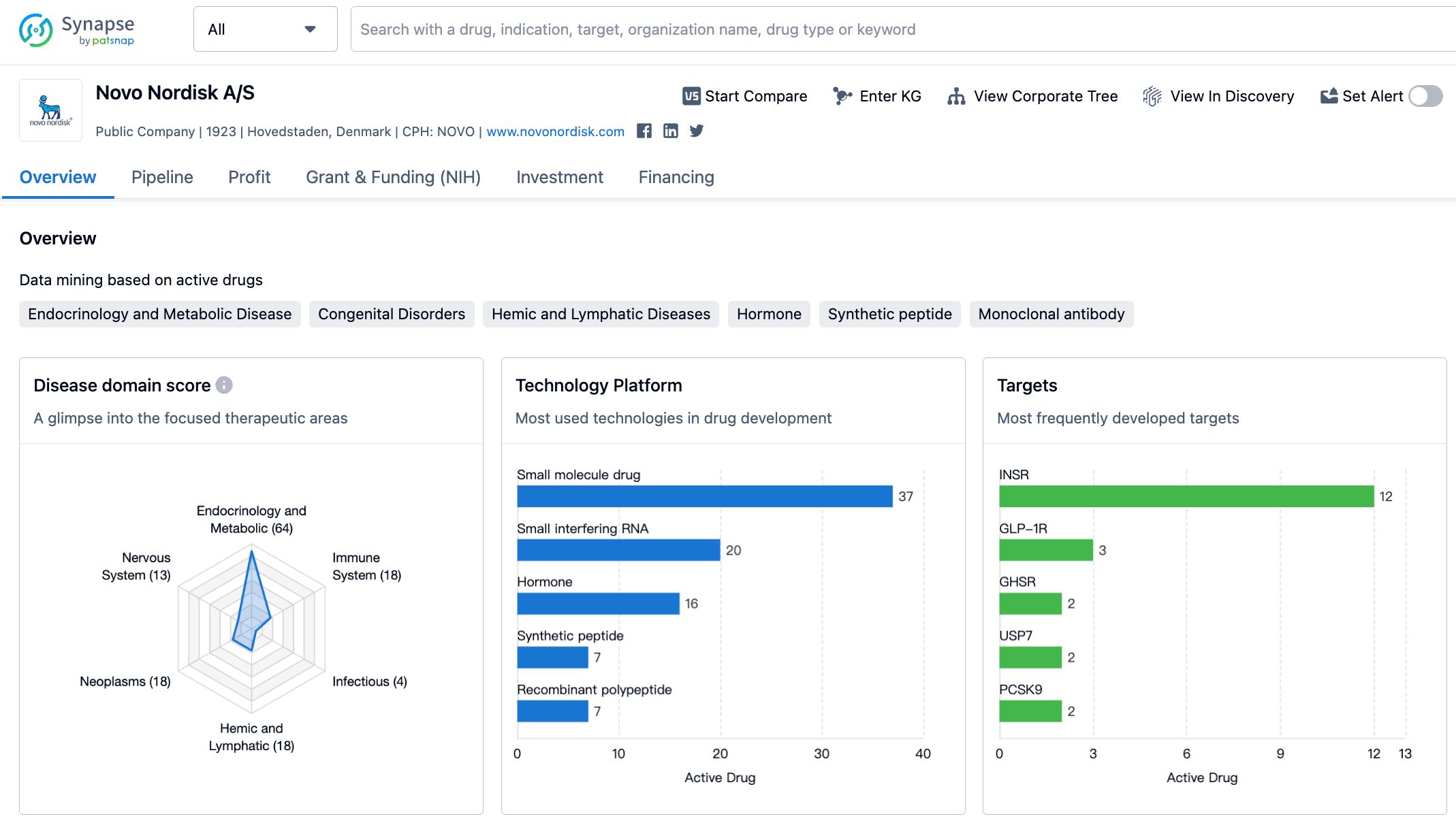
Latest Competitive Analysis of Novo Nordisk Drug Pipeline
Novo Nordisk has nearly succeeded by relying on one metabolic domain and leveraging the power of GLP-1 alone, raising its market value from 100 billion to over 400 billion, reaching unprecedented heights on the centenary of its founding.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of Novo Nordisk.
Despite the massive growth comparable to Eli Lilly, Novo Nordisk primarily owes its success to its performance, with LTM P/E multiples rising from 40x to 45x.
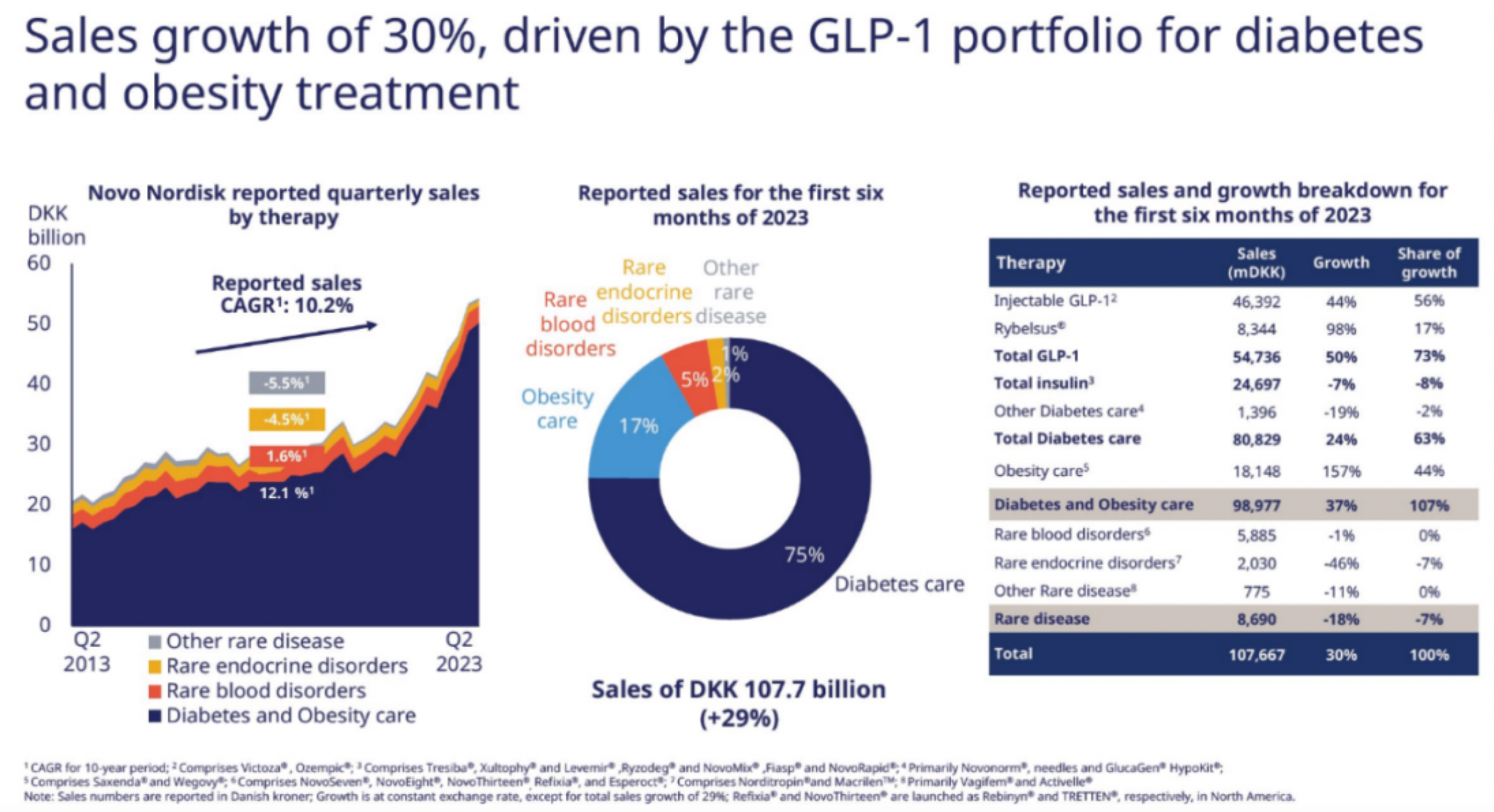
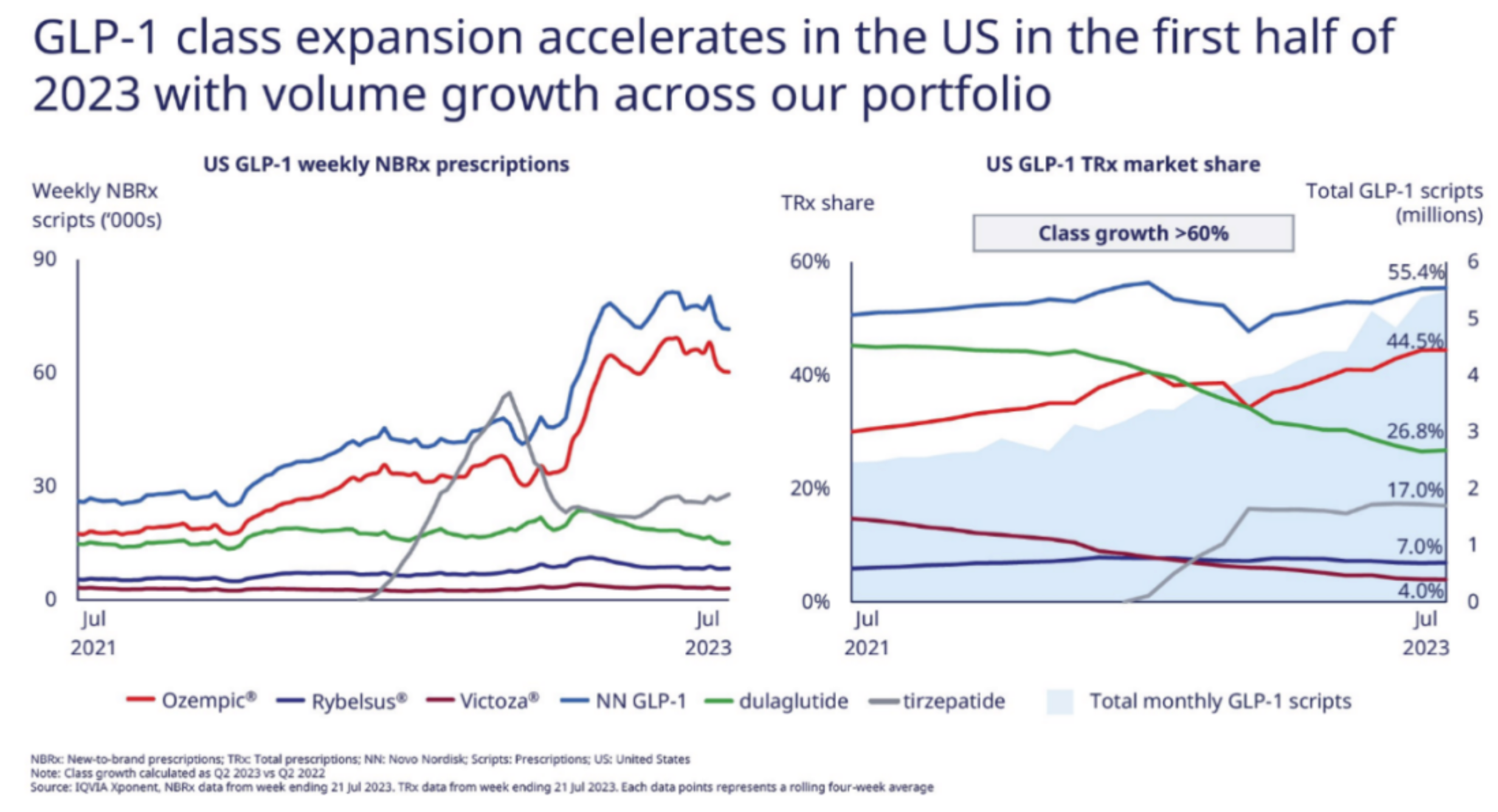
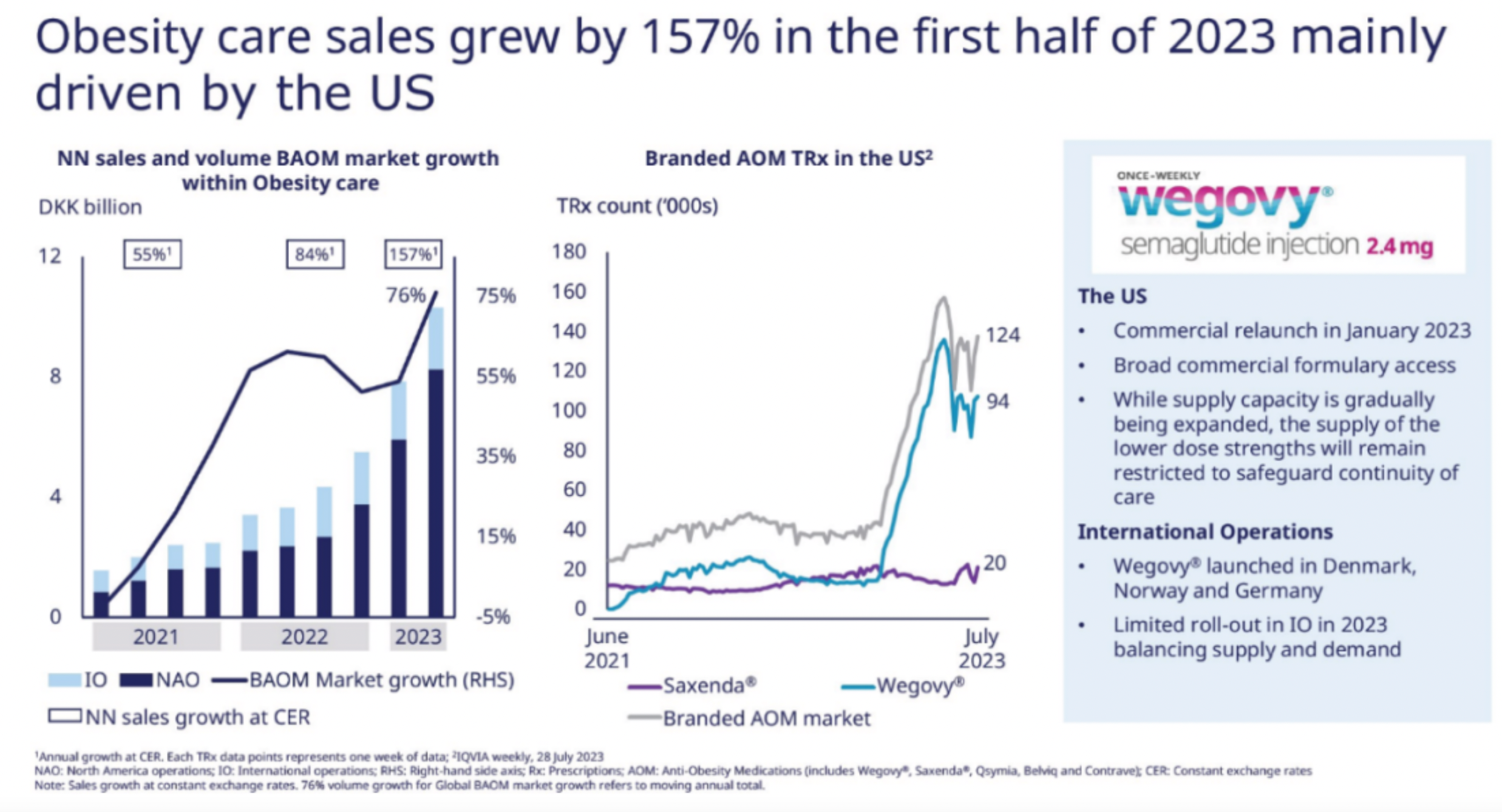
The semi-annual sales of Semaglutide reached $9.2 billion, providing Novo Nordisk with a rare growth rate among large pharmaceutical enterprises. Though the rapid growth of Tirzepatide is evident, against the backdrop of its base, S-drug's prescription volume and market share still hold a dominant advantage.
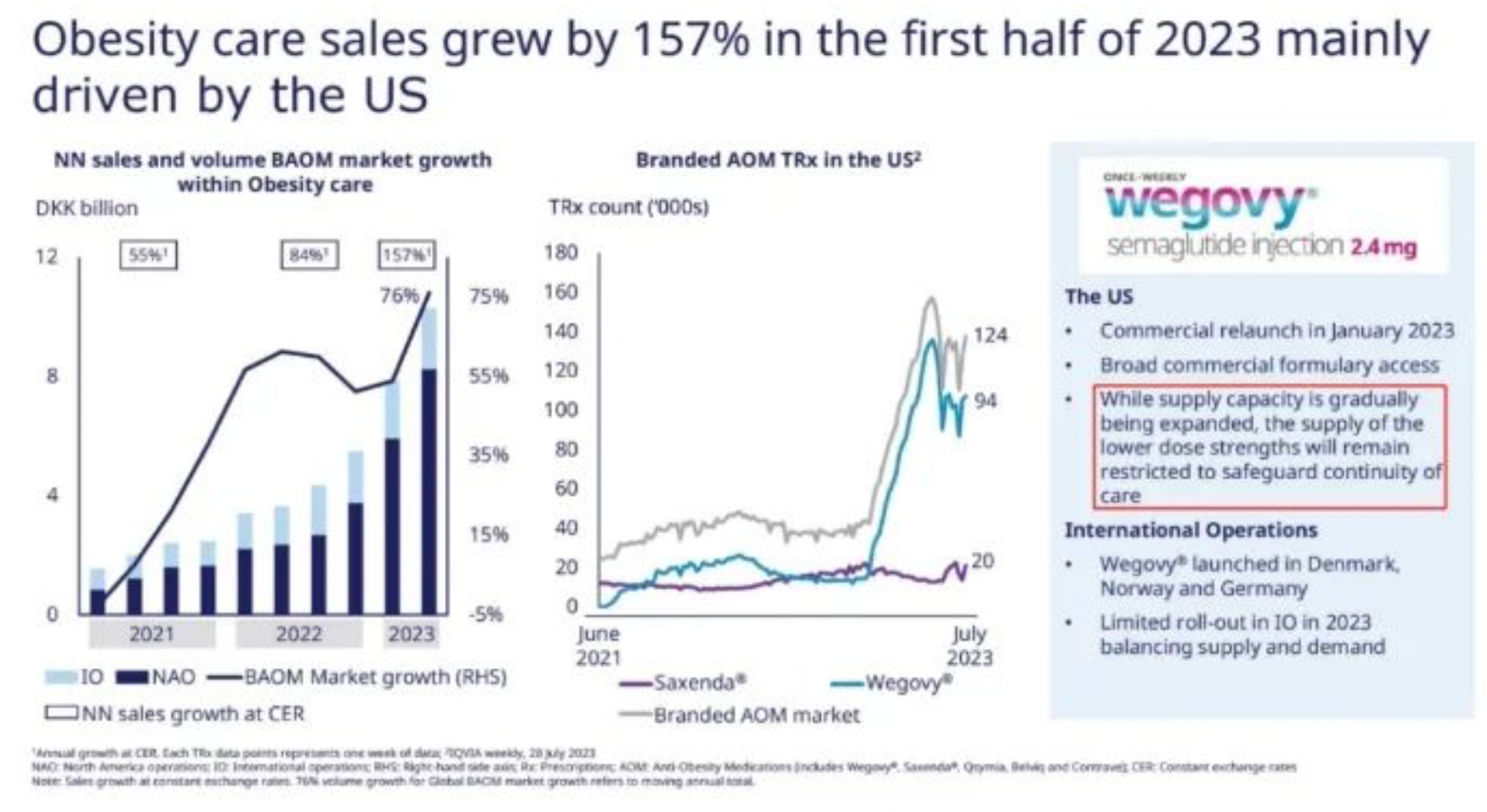
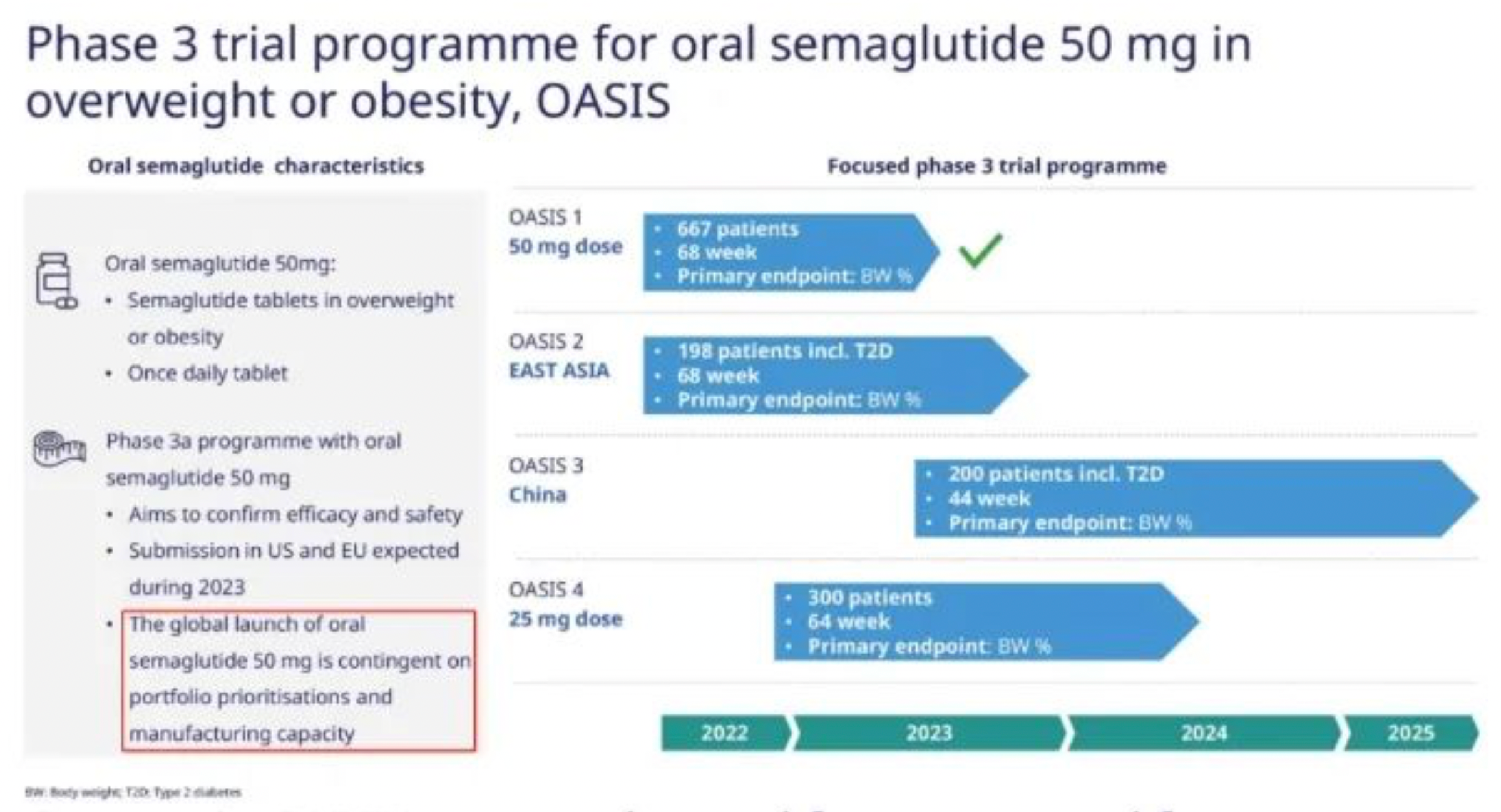
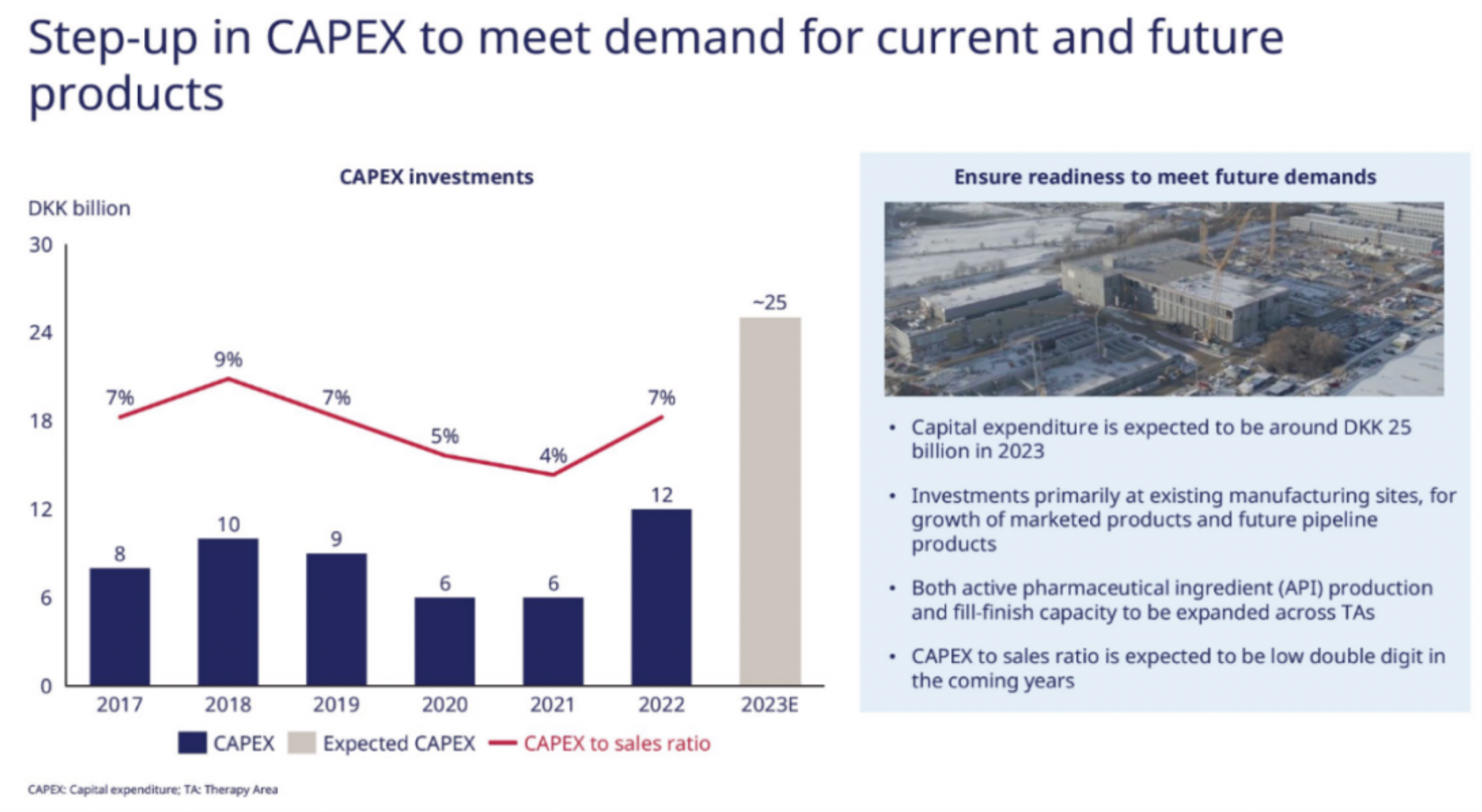
For Novo Nordisk, the main constraint on performance remains to be the severe lack of production capacity. The Belgium based CDMO formulation factory of Catalent, which had previously been shut down by the FDA, is expected to gradually restore production from the end of 2022. This led to Novo Nordisk being forced to apply the brakes on its promotion of Wegovy, even specifically adopting a wait-and-see attitude for the oral 50mg dosage. Additionally, the production capacity of APIs is expanding at a swift pace, with the company undergoing significant construction in both Denmark and the U.S.
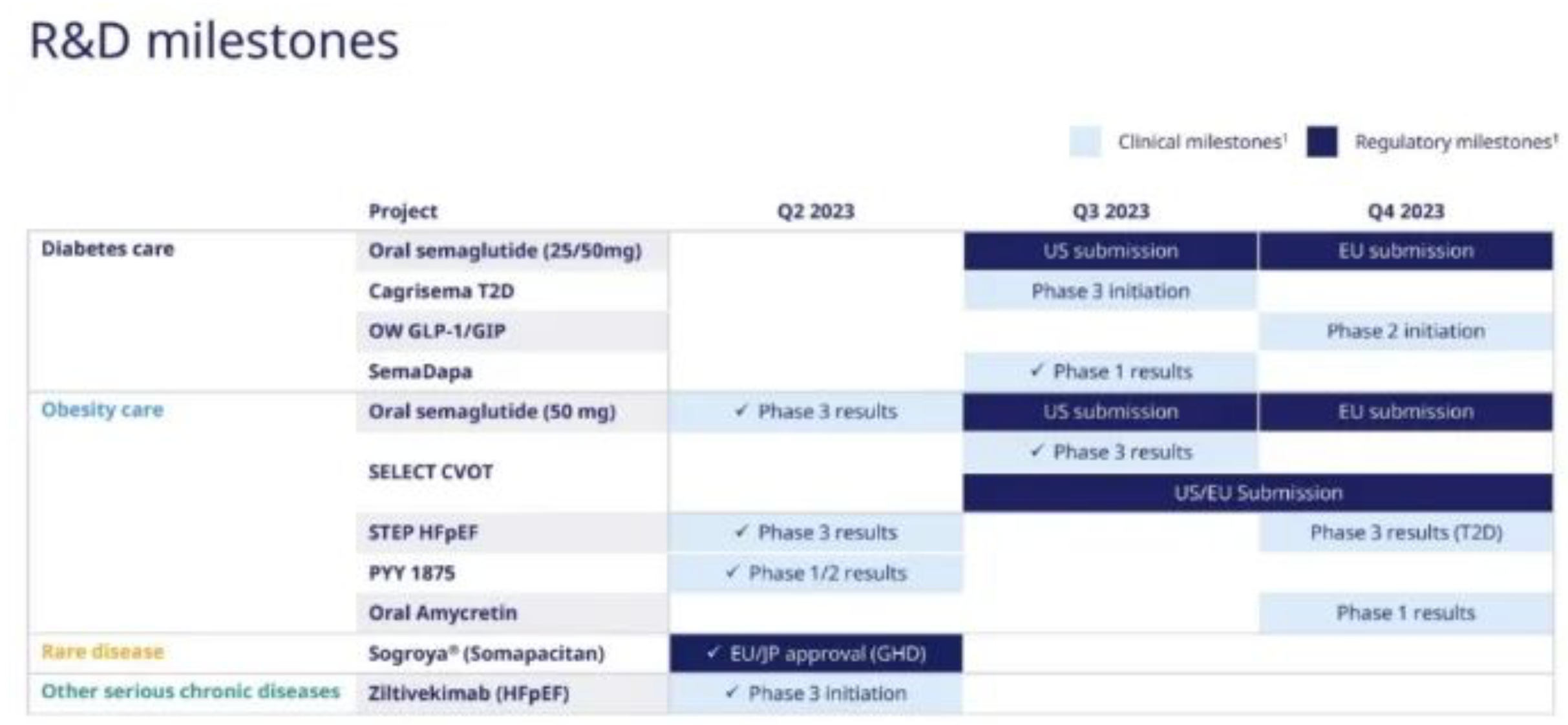
Of course, the most important R&D progress is Semaglutide's Phase III clinical results for heart failure and cardiovascular risk prevention, as well as the Phase III clinical results of the 50mg oral dosage form. Besides this, the weekly insulin product Icodec has submitted its BLA in Europe and the U.S., the extended-release growth hormone Somapacitan was approved for paediatric indications, and the acquisition of Inversago for the new weight loss drug INV-202, all are worth noting. The bad news includes the receipt of a CRL for the TFPI monoclonal antibody Concizumab for the treatment of hemophilia, the termination of the Phase II PYY1875, and the disappearance of the small molecule PCSK9 and long-acting GDF15 from the pipeline.