Latest Developments in the Research and Development of Angiotensin II Receptor Antagonists
The Angiotensin II receptor plays a crucial role in the human body by regulating blood pressure and fluid balance. It is a key component of the renin-angiotensin-aldosterone system, which helps maintain homeostasis. Angiotensin II, a hormone, binds to these receptors, leading to vasoconstriction, increased blood pressure, and the release of aldosterone, which promotes sodium and water retention. This receptor is also involved in various physiological processes, including cell growth, inflammation, and oxidative stress.
Understanding the role of the Angiotensin II receptor is essential for developing drugs that target this pathway to treat conditions such as hypertension, heart failure, and kidney diseases
Angiotensin II receptor Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 8 Sep 2023, there are a total of 280 Angiotensin II receptor drugs worldwide, from 219 organizations, covering 83 indications, and conducting 2889 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.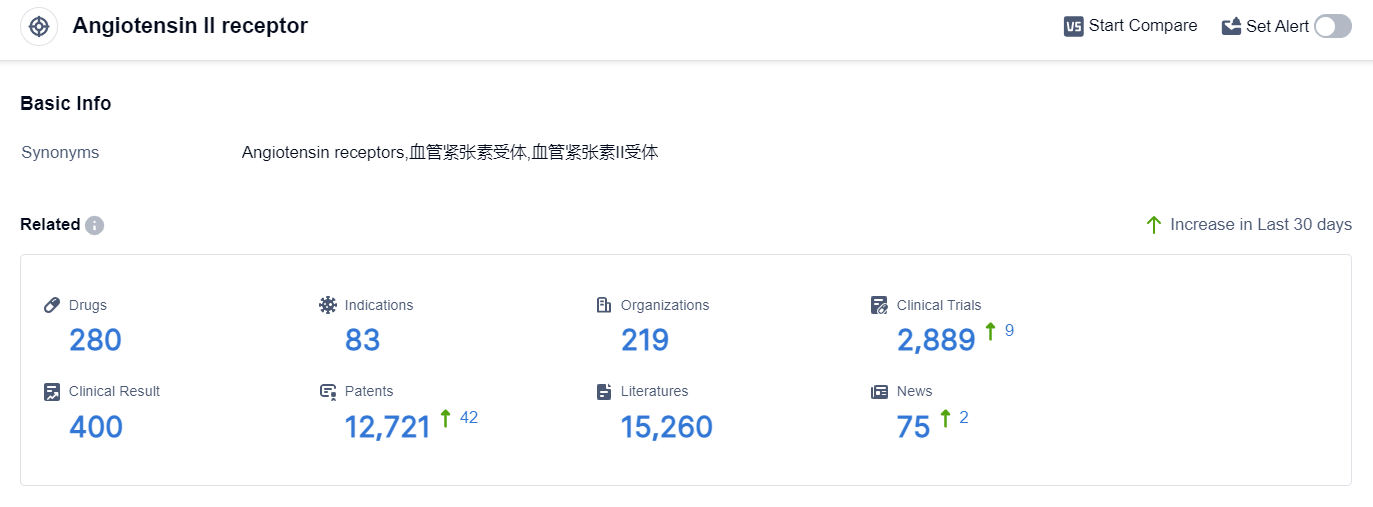
The target Angiotensin II receptor in the pharmaceutical industry has seen significant development and competition. Takeda Pharmaceutical Co., Ltd., Boryung Corp., Novartis AG, and Daiichi Sankyo Co., Ltd. are among the companies with the highest stage of development on this target.
Hypertension is the most common approved indication, and small molecule drugs are the most rapidly progressing drug type. South Korea, the United States, and the European Union are leading in terms of development, with China also making progress.
The future development of the target Angiotensin II receptor is expected to continue with intense competition and advancements in various countries and companies.
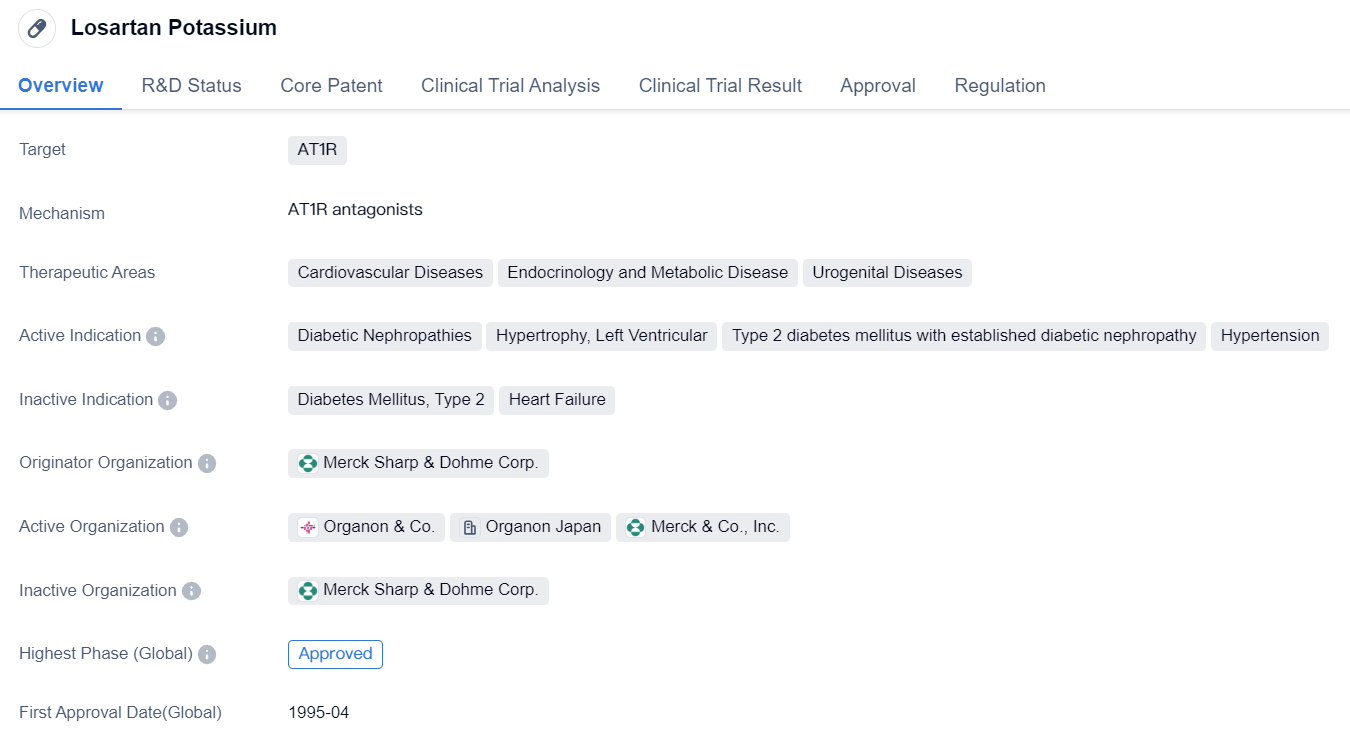
The first highly selective Angiotensin II receptor antagonists: Losartan Potassium
Losartan Potassium is a small molecule drug that targets angiotensin II type 1 receptor (AT1R), which is involved in various physiological processes related to cardiovascular diseases, endocrinology and metabolic diseases, and urogenital diseases. It has been approved for the treatment of several conditions, including diabetic nephropathies, left ventricular hypertrophy, type 2 diabetes mellitus with established diabetic nephropathy, and hypertension.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The drug was developed by Merck Sharp & Dohme Corp., a renowned pharmaceutical organization. It received its first approval in the United States in April 1995, making it a well-established medication in the market. Losartan Potassium has also obtained approval in China, indicating its global recognition and acceptance.
The therapeutic areas targeted by Losartan Potassium reflect its broad applicability in treating various diseases. Cardiovascular diseases, such as hypertension and left ventricular hypertrophy, are prevalent worldwide and contribute significantly to morbidity and mortality rates. Endocrinology and metabolic diseases, including type 2 diabetes mellitus and diabetic nephropathies, are also widespread and require effective treatment options to manage their complications. Urogenital diseases encompass a range of conditions affecting the urinary and reproductive systems, further expanding the potential applications of Losartan Potassium.
Overall, Losartan Potassium is a small molecule drug that targets AT1R and has been approved for the treatment of cardiovascular diseases, endocrinology and metabolic diseases, and urogenital diseases. Developed by Merck Sharp & Dohme Corp., it has received approvals in both the United States and China. Its orphan drug status indicates its potential to address unmet medical needs, and its therapeutic areas highlight its versatility in treating various conditions. With its long history of use since 1995, Losartan Potassium has established itself as a trusted medication in the pharmaceutical industry.
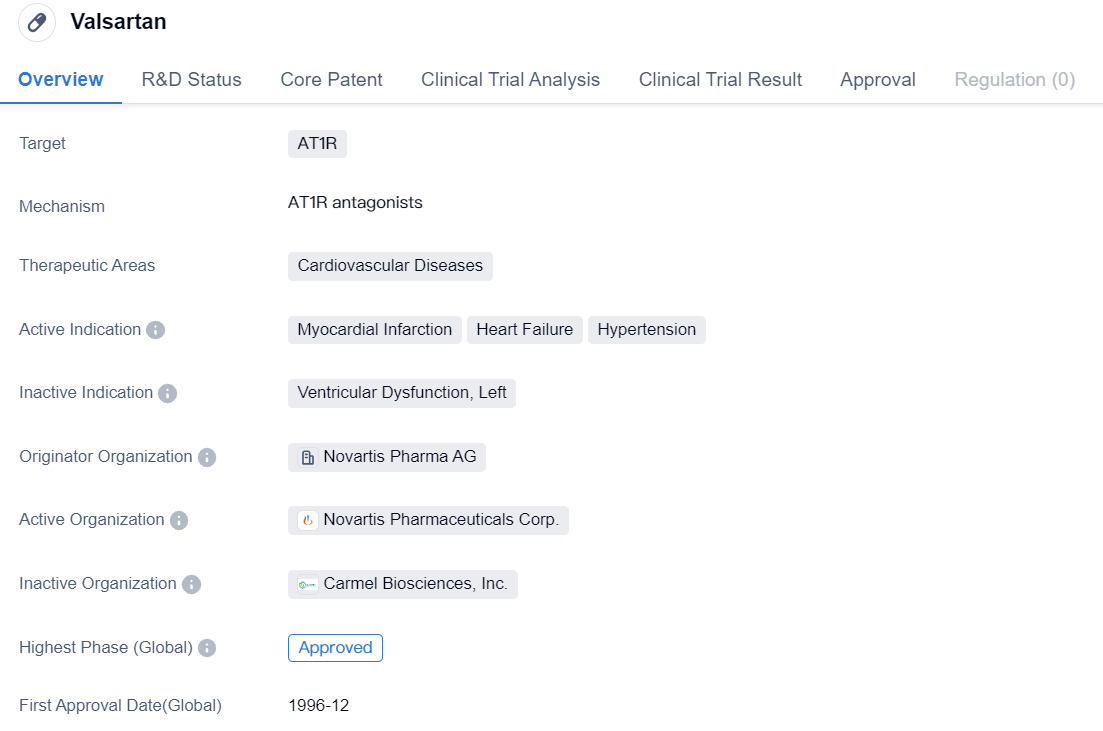
Second Generation Non-peptide Angiotensin II Receptor Antagonists: Valsartan
Valsartan is a small molecule drug that falls under the therapeutic area of cardiovascular diseases. It specifically targets AT1R, which is involved in regulating blood pressure and fluid balance. The drug has been approved for use in treating various cardiovascular conditions, including myocardial infarction, heart failure, and hypertension.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Novartis Pharma AG is the originator organization of Valsartan. The drug has reached the highest phase of approval both globally and in China. It received its first approval in December 1996 in the United States.
As a small molecule drug, Valsartan is designed to interact with specific receptors in the body to produce a therapeutic effect. In this case, it targets AT1R, which is a receptor involved in the regulation of blood pressure. By blocking the action of AT1R, Valsartan helps to relax and widen blood vessels, reducing blood pressure and improving blood flow.
The approval of Valsartan for the treatment of myocardial infarction, heart failure, and hypertension highlights its effectiveness in managing these cardiovascular conditions. Myocardial infarction, commonly known as a heart attack, occurs when blood flow to the heart is blocked, leading to tissue damage. Heart failure, on the other hand, is a chronic condition where the heart is unable to pump blood effectively. Hypertension, or high blood pressure, is a common condition that increases the risk of cardiovascular diseases.
In summary, Valsartan is a small molecule drug that targets AT1R and is used in the treatment of cardiovascular diseases. Its approval for myocardial infarction, heart failure, and hypertension showcases its therapeutic potential. Developed by Novartis Pharma AG, Valsartan has achieved the highest phase of approval globally and in China, making it a significant drug in the field of biomedicine.