Latest Developments in the Research and Development of C5 Inhibitor
C5, or the fifth component of complement, plays a crucial role in the human body's immune system. It is a protein that is part of the complement system, a group of proteins that work together to defend against infections and promote inflammation. C5 is involved in the activation of the complement cascade, which leads to the destruction of foreign pathogens, such as bacteria and viruses. Additionally, C5 is also implicated in various inflammatory diseases and disorders, making it an important target for pharmaceutical interventions aimed at modulating the immune response and treating related conditions.
C5 Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 8 Sep 2023, there are a total of 45 C5 drugs worldwide, from 66 organizations, covering 78 indications, and conducting 373 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.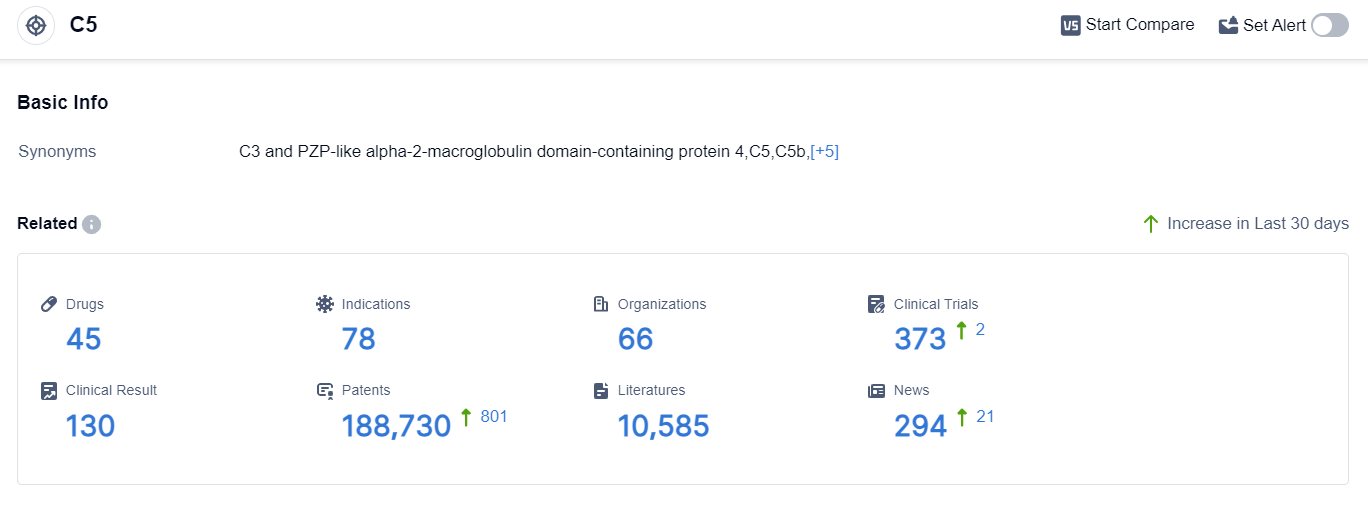
Based on the analysis of the data provided, the current competitive landscape of target C5 is characterized by the presence of companies such as AstraZeneca PLC, InflaRx NV, and Regeneron Pharmaceuticals, Inc. These companies are leading the way in terms of R&D progress and have a significant number of drugs in various stages of development.
The indications for drugs under target C5 cover a wide range of diseases and conditions, indicating the potential of this target in addressing various medical needs. Monoclonal antibodies and biosimilars are the drug types that are progressing most rapidly, suggesting intense competition and a focus on innovation.
The United States, the European Union, and Japan are the countries/locations that are developing fastest under target C5. However, China is also making progress and is likely to play a significant role in the future development of this target.
Overall, target C5 presents a promising opportunity for companies in the pharmaceutical industry. The competition is intense, but the potential for developing innovative drugs to address various medical needs is significant. Continued research and development efforts, especially in monoclonal antibodies and biosimilars, will be crucial for success in this market.
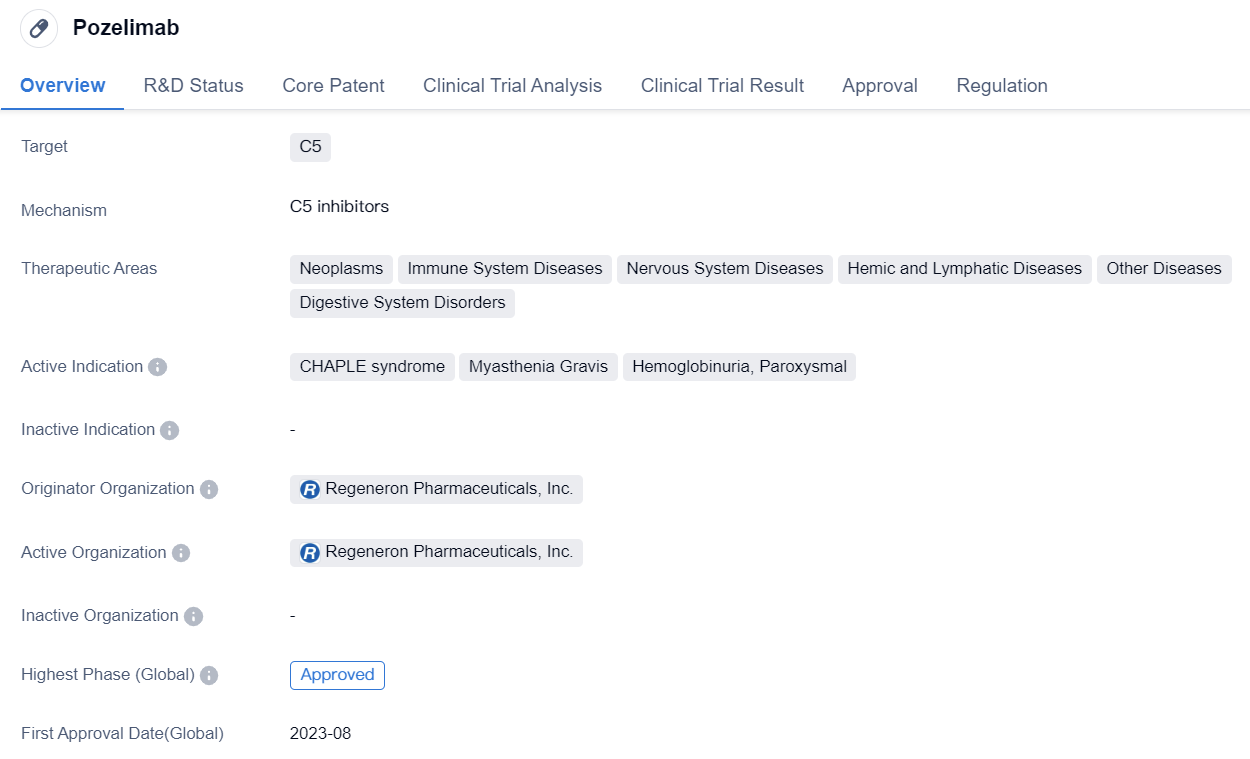
Key Drug: Pozelimab
Pozelimabis a monoclonal antibody drug developed by Regeneron Pharmaceuticals, Inc. It specifically targets the C5 protein and is primarily used in the treatment of various diseases related to the immune system, nervous system, hemic and lymphatic system, digestive system, and other diseases. The drug has shown promising results in clinical trials and has received approval for use in the United States.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The therapeutic areas of Pozelimab include neoplasms, immune system diseases, nervous system diseases, hemic and lymphatic diseases, other diseases, and digestive system disorders. This indicates that the drug has potential applications in a wide range of medical conditions.
Pozelimab has been particularly effective in treating CHAPLE syndrome, myasthenia gravis, and hemoglobinuria, paroxysmal. These conditions are characterized by dysfunctions in the immune system and can have severe consequences if left untreated. The drug's ability to target the C5 protein makes it a valuable tool in managing these diseases.
Pozelimab received its first approval in the United States in August 2023. This indicates that the drug has met the necessary regulatory requirements and has been deemed safe and effective for use in patients. Additionally, Pozelimab has been granted orphan drug status and priority review, highlighting its potential to address unmet medical needs and expedite the approval process.
In conclusion, Pozelimab is a monoclonal antibody drug developed by Regeneron Pharmaceuticals, Inc. It targets the C5 protein and has shown promising results in treating various diseases related to the immune system, nervous system, hemic and lymphatic system, digestive system, and other diseases. The drug has received approval in the United States and holds orphan drug status and priority review. Pozelimab's approval and therapeutic potential make it a significant advancement in the field of biomedicine.
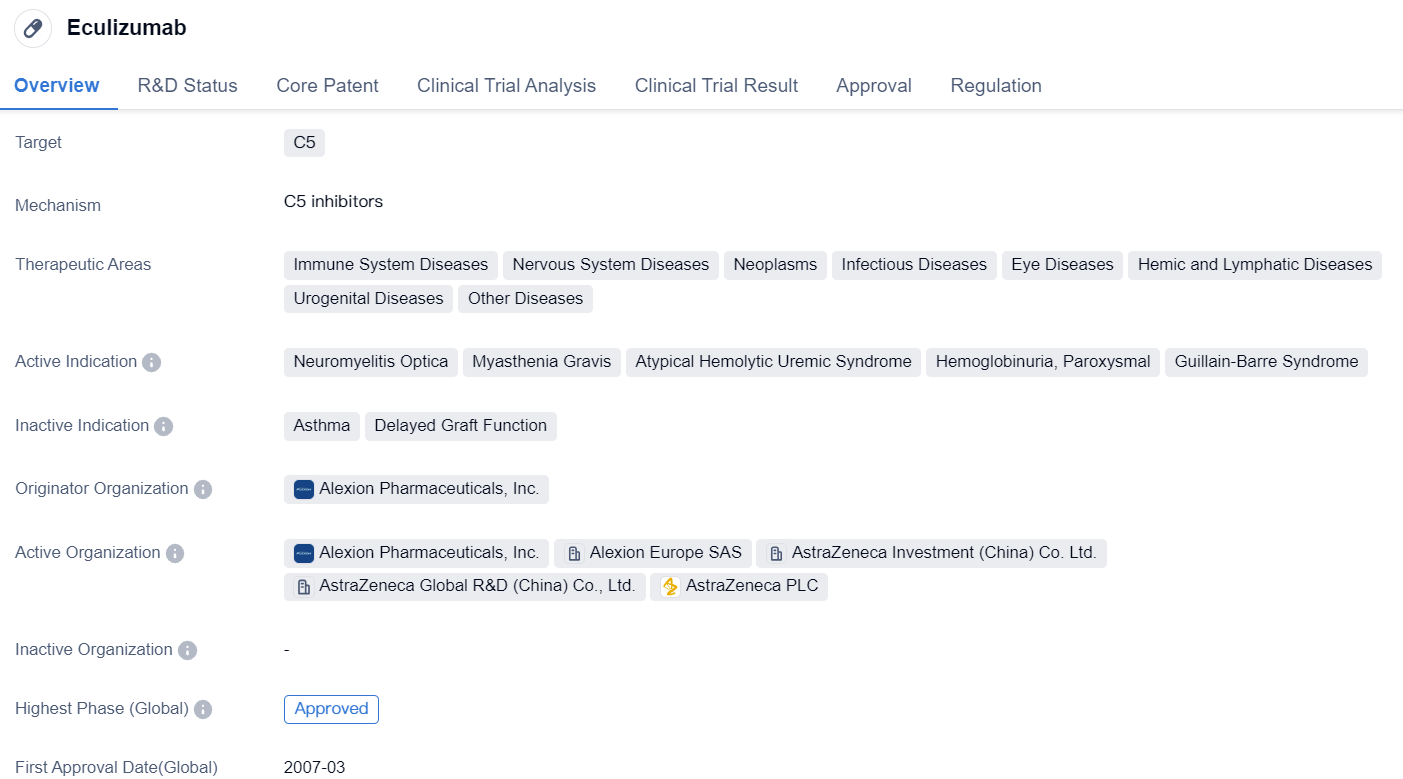
Eculizumab
Eculizumab is a monoclonal antibody drug that targets the C5 protein. It has been approved for use in various therapeutic areas, including immune system diseases, nervous system diseases, neoplasms, infectious diseases, eye diseases, hemic and lymphatic diseases, urogenital diseases, and other diseases. The drug is indicated for the treatment of several conditions, including Neuromyelitis Optica, Myasthenia Gravis, Atypical Hemolytic Uremic Syndrome, Hemoglobinuria Paroxysmal, and Guillain-Barre Syndrome.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Eculizumab was developed by Alexion Pharmaceuticals, Inc., a pharmaceutical company specializing in rare diseases. The drug has received approval in multiple countries, with its first approval granted in the United States in March 2007. It has also received priority review and orphan drug designation, indicating its potential to address unmet medical needs for rare diseases.
As a monoclonal antibody, eculizumab works by inhibiting the C5 protein, which plays a crucial role in the complement system of the immune system. By blocking C5, the drug helps to prevent the destruction of red blood cells and the subsequent damage to various organs and tissues.
The approval of eculizumab in multiple therapeutic areas highlights its versatility and potential to address a wide range of diseases. Its approval for rare diseases, such as Atypical Hemolytic Uremic Syndrome and Hemoglobinuria Paroxysmal, demonstrates its effectiveness in treating conditions with limited treatment options.
The drug's approval in China, as well as its global approval, indicates its potential for international markets. This could open up opportunities for expansion and increased accessibility to patients in need.
Overall, eculizumab is a monoclonal antibody drug that targets the C5 protein and has been approved for various therapeutic areas. Its approval in multiple countries, including the United States and China, along with its priority review and orphan drug designation, highlight its potential to address unmet medical needs in rare diseases.