Is Dasiglucagon approved by the FDA?
Dasiglucagon, marketed under the brand names Zegalogue Autoinjector and Zegalogue Prefilled Syringe, is an injectable medication used to treat severe hypoglycemia (very low blood sugar) in adults and children aged six years and older with diabetes.Dasiglucagon (Zegalogue) was approved by the U.S. Food and Drug Administration (FDA) on March 22, 2021. This approval introduced a new emergency treatment option for managing severe hypoglycemia, providing a fast-acting solution for diabetic patients in critical situations.
Mechanism of Action
Dasiglucagon works by stimulating the liver to release stored glucose into the bloodstream, thereby rapidly increasing blood sugar levels. It is administered subcutaneously (under the skin) and is designed for use in emergency situations where immediate action is required to prevent severe complications from hypoglycemia, such as seizures, coma, or death.
Usage and Administration
Dosage Form: Dasiglucagon is available as a subcutaneous solution with a concentration of 0.6 mg/0.6 mL.
Dosage Instructions:
- Adults and Children (6 years and older): The recommended dose is 0.6 mg administered subcutaneously into the lower abdomen, buttocks, thigh, or outer upper arm. If there is no response within 15 minutes, a second dose of 0.6 mg may be given.
Administration:
- Be familiar with the instructions for using dasiglucagon injections. Caregivers should know how to administer the medication before an emergency occurs.
- After an injection, the patient should consume a fast-acting source of sugar, followed by a snack or small meal to stabilize blood sugar levels.
Side Effects and Warnings
Common Side Effects:
- Nausea
- Vomiting
- Diarrhea
- Headache
- Injection site pain
Serious Side Effects:
- Severe headache
- Blurred vision
- Rapid or pounding heartbeat
- Ongoing low blood sugar symptoms (e.g., dizziness, sweating, fast heart rate)
Warnings:
- Do not use dasiglucagon if you have an insulinoma (pancreatic tumor) or pheochromocytoma (adrenal gland tumor).
- Be cautious if you have chronic low blood sugar, adrenal gland problems, or a latex allergy.
- Follow your doctor's instructions closely to manage your diet, medication, and exercise routines to prevent severe hypoglycemia.
Storage:
- Store dasiglucagon in the refrigerator, away from the cooling element. Do not freeze.
- It can also be stored at room temperature for up to 12 months but must be used within this period.
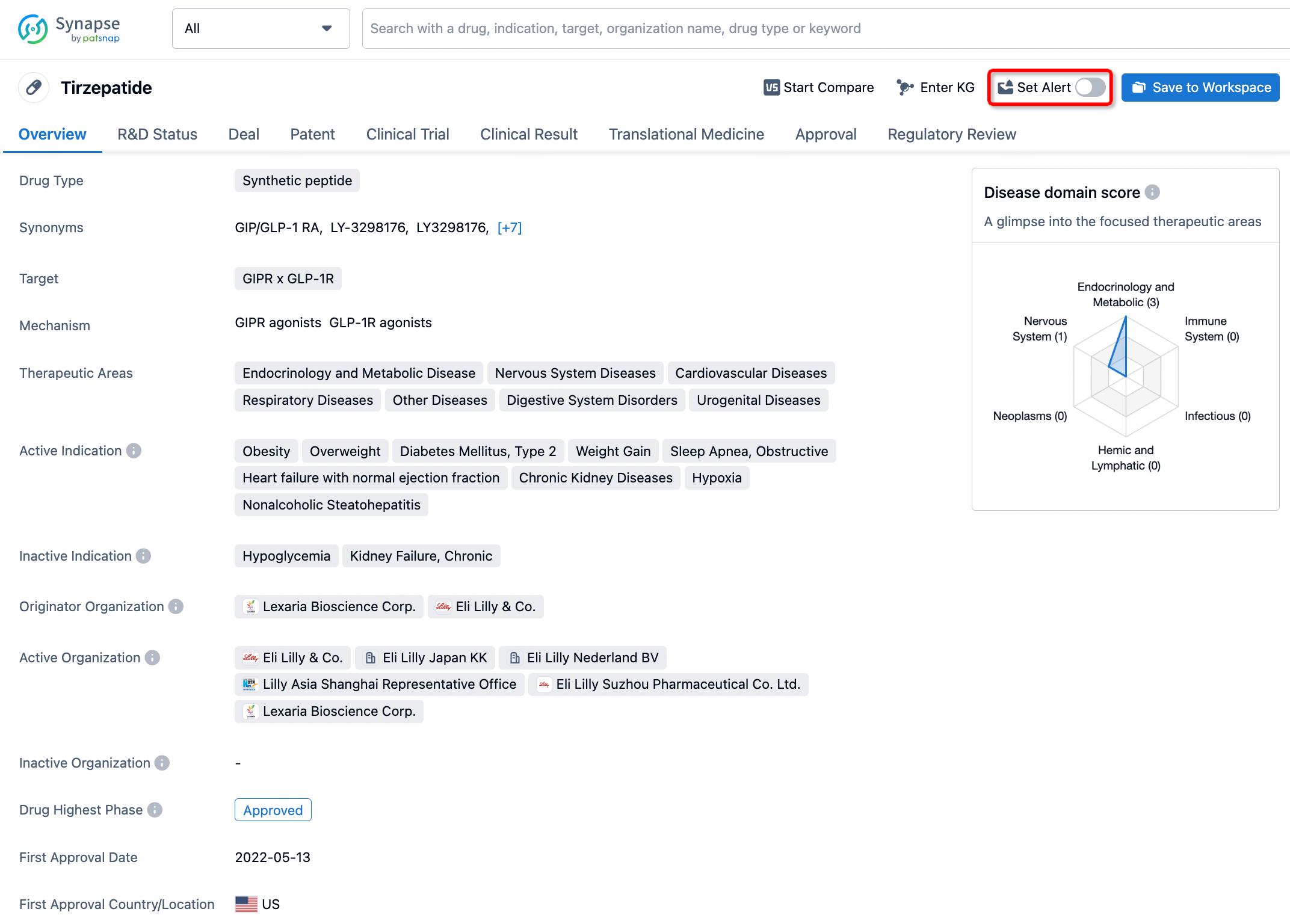
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!