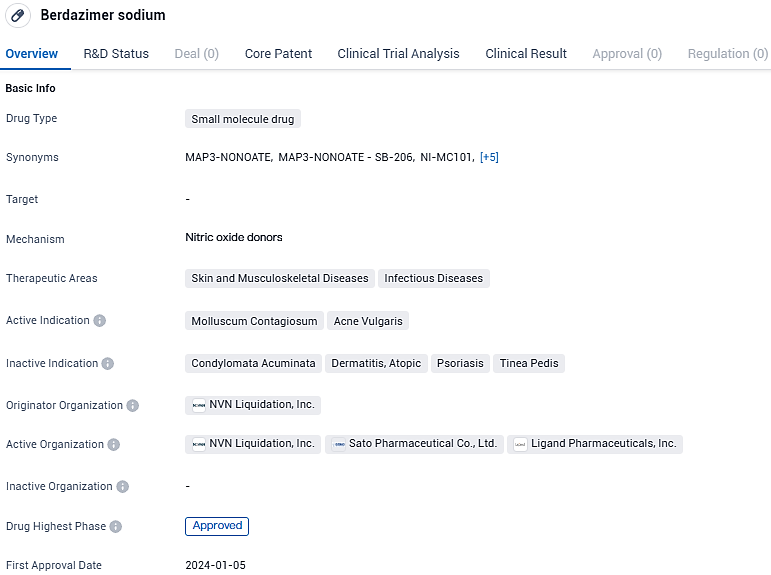
Ligand Pharmaceuticals Inc. announces ZELSUVMI™ FDA approval for treating molluscum contagiosum in adults and children over 1 year old
Ligand Pharmaceuticals Incorporated has declared the receipt of approval from the FDA for their product ZELSUVMI™ (berdazimer topical gel, 10.3%), which is now authorized for the medical management of molluscum contagiosum in both adults and children from the age of one year and upwards. This marks the initial instance of a new medicinal solution sanctioned by the FDA specifically designed to combat infections caused by molluscum.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
ZELSUVMI has emerged as the inaugural non-hospital prescription solution for treating a widespread viral skin condition. Unlike before, patients, their family members, or individuals providing care can apply the formulation within the comfort of their homes rather than seeking a healthcare facility. This represents a significant shift in the management of this infectious disease.
Dr. Mark D. Kaufmann, MD, FAAD, a reputed Clinical Professor of Dermatology at Mount Sinai's Icahn School of Medicine in New York and a former head of the American Academy of Dermatology, expressed his enthusiasm over the authorization of ZELSUVMI. He recognized it as a milestone, adding a new dimension to how molluscum is tackled by providing patients and caregivers with a potent topical medicine they can administer themselves. Dr. Kaufmann eagerly anticipates integrating this innovative treatment into his practice for individuals with molluscum.
Characterized by pearly, dome-shaped nodules with a distinctive dimple at the center, molluscum contagiosum is a highly transmissible dermatological condition. In the United States alone, it is estimated that 6 million – mainly pediatric – cases emerge annually. Despite the high prevalence, a substantial portion of these cases receive no intervention. Given that the viral disease has the potential to proliferate across the skin and to other individuals, it's considered imperative to address the presenting symptoms.
Dr. Stephen W. Stripling, MD, a Pediatrician, investigator in clinical research, and authority on molluscum contagiosum, welcomed the increased focus on the condition and advocated for the new therapeutic pathway. Highlighting a shift from the conventional observational stance, he celebrated the availability of an effective at-home treatment for healthcare providers in primary care settings.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
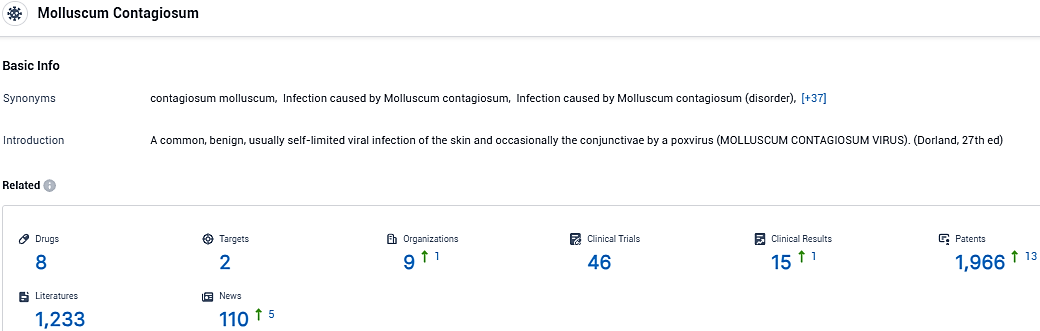
According to the data provided by the Synapse Database, As of January 10, 2024, there are 8 investigational drugs for Molluscum Contagiosum, including 2 targets, 9 R&D institutions involved, with related clinical trials reaching 46, and as many as 1966 patents.
ZELSUVMI (berdazimer topical gel, 10.3%) is a nitric oxide releasing agent indicated for the topical treatment of molluscum contagiosum in adults and pediatric patients one year of age and older. ZELSUVMI is expected to be available in the United States in the second half of 2024.