Lilly Plans to Purchase POINT Biopharma, Enhancing Cancer Treatment Abilities with Advanced Radioligand Therapies
Eli Lilly along with POINT Biopharma Global, Inc. have declared a final agreement where Lilly will take over POINT, a company specializing in radiopharmaceuticals. POINT has a series of clinical and preclinical-stage radioligand therapies under development, all aimed at combating cancer.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Radioligand therapy has the potential to precisely attack cancer, by connecting a radioisotope to a directive molecule. This process delivers radiation straight to cancer cells, permitting notable anti-cancer efficacy and concurrently lessening the effect on healthy tissue.
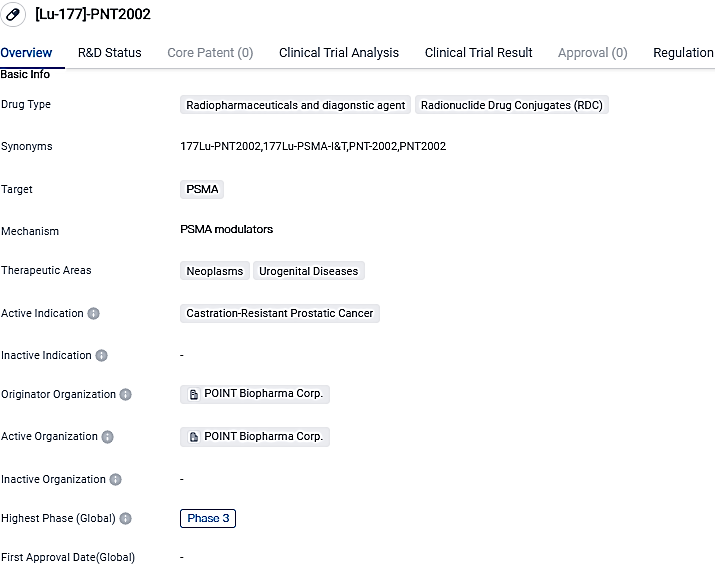
The leading programs of POINT are in advanced stages of development. PNT2002, a PSMA targeted radioligand therapy, is currently being developed for patients suffering from metastatic castration-resistant prostate cancer post the progression on hormonal treatment. The primary data from this research is anticipated in the end phase of 2023.
PNT2003, a somatostatin receptor targeted radioligand therapy, is being developed for the treatment of patients diagnosed with gastroenteropancreatic neuroendocrine tumors. POINT operates additional programs in initial phases of clinical and preclinical development. These facilities will be exploited in conjunction with POINT's extensive network of supply chain associates for procuring radioisotopes and their precursors.
"In recent times, we have witnessed how effectively sophisticated radiopharmaceuticals can yield significant results for cancer patients and quickly incorporate into care standards, however, the industry is still in its preliminary phase of the impact they might ultimately supply," stated Jacob Van Naarden, President of Loxo@Lilly, the cancer unit of Eli Lilly and Company.
Jacob Van Naarden followed, "We are enthusiastic about the potential of this emerging technique and view POINT's acquisition as a prelude to our investment in the development of several significant radioligand medicines for difficult-to-treat cancers. We eagerly anticipate the arrival of colleagues from POINT to Lilly and look forward to combining our successes to generate a pipeline of new, significant radioligand treatments for patients."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
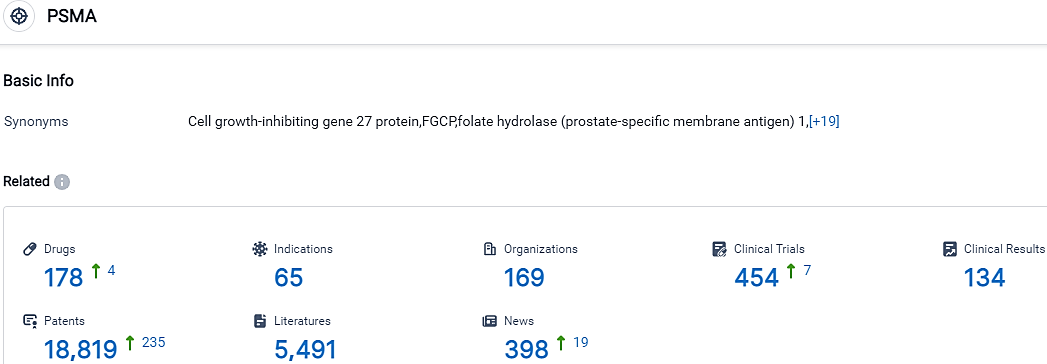
According to the data provided by the Synapse Database, As of October 11, 2023, there are 178 investigational drugs for the PSMA target, including 65 indications, 169 R&D institutions involved, with related clinical trials reaching 454,and as many as 18819 patents.
[Lu-177]-PNT2002 shows promise as a targeted therapy for patients with Castration-Resistant Prostatic Cancer. The drug's specific targeting of PSMA, combined with its radiopharmaceutical properties, may offer a more effective and precise treatment approach. The ongoing Phase 3 trials will provide further insights into the drug's safety and efficacy, potentially paving the way for its approval and commercialization.