OliX Pharmaceuticals reports promising preliminary safety and efficacy data from a Phase 1 trial of OLX10212 for age-related macular degeneration
OliX Pharmaceuticals, Inc., an innovative pioneer in the field of RNA interference (RNAi) drugs, has recently disclosed encouraging outcomes from an initial Phase 1 clinical trial. This study assessed the risk profile and patient acceptance of their drug compound, OLX10212, specifically designed to combat Age-Related Macular Degeneration.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Age-related macular degeneration (AMD) stands as the principal culprit behind vision loss in developed nations, afflicting over 170 million individuals globally. The treatments currently on the market typically fail to reverse the damage caused by AMD or halt its advancement effectively.
The advent of small interfering RNAs (siRNAs) signifies a groundbreaking direction in AMD therapy. Compounds such as OLX10212 have demonstrated promising results in preclinical trials for both prevalent types of the condition: neovascular and atrophic (dry) AMD. These siRNAs are designed to intercept crucial biological processes implicated in the onset and progression of AMD, offering a fresh avenue for patient care.
This initial phase clinical trial is organized across multiple sites and utilizes a single ascending dose design to ascertain the safety and tolerability of OLX10212 in individuals with neovascular AMD. The pivotal outcomes of this research encompassed monitoring safety and tolerability linked to the administration of OLX10212 via intravitreal injections.
Observations in the trial indicated an absence of inflammatory responses and maintained stability of the internal ocular environment among all participants. Furthermore, no systemic implications were reported. Although some patients experienced temporary, mild side effects relative to the injection process, these were anticipated for intravitreal treatments. Crucially, the trial was successful in determining appropriate dosing for further explorations of OLX10212's effectiveness.
In summary, the trial fulfilled its intended objectives, establishing a firm foundation for the continuing investigation of OLX10212 in treating AMD based on the safety, tolerability, and initial indications of visual acuity enhancement.
Providing his expert insight, Demetrios G. Vavvas, M.D., Ph.D.—head of the Retina Service at Harvard Medical School and a consultant for OliX Pharmaceuticals—expressed optimism: “The emergence of asymmetric siRNA within clinical settings marks a significant advancement for eye care medications. Anticipating the future phases of clinical research, we are hopeful about the potential impact of this technology.”
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
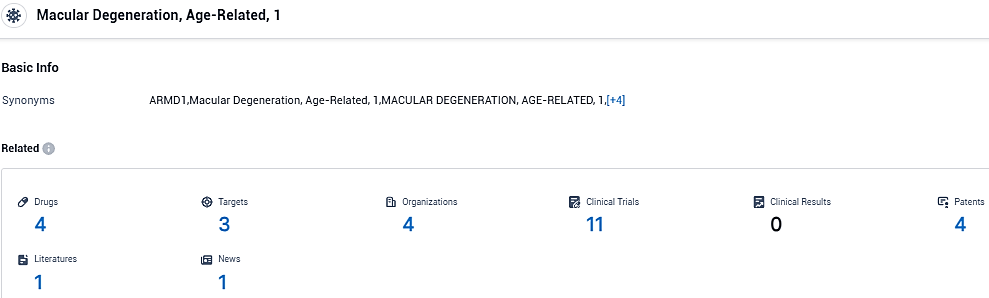
According to the data provided by the Synapse Database, As of December 6, 2023, there are 4 investigational drugs for Macular Degeneration, Age-Related, 1, including 3 targets, 4 R&D institutions involved, with related clinical trials reaching 11, and as many as 4 patents.
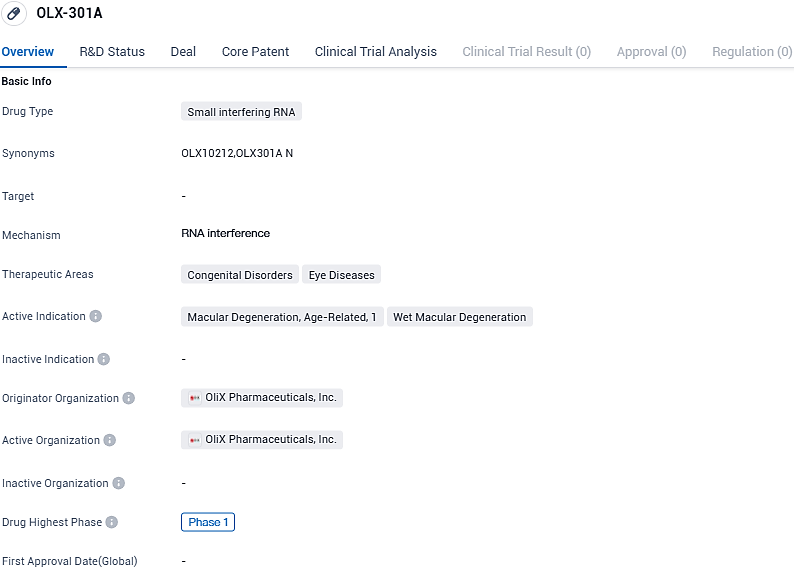
OLX-301A is a siRNA drug developed for the treatment of macular degeneration, including age-related and wet macular degeneration. With its small interfering RNA mechanism, OLX-301A has the potential to provide targeted therapy for these eye diseases. However, as it is currently in Phase 1 of development, further clinical trials and evaluations are required to determine its efficacy and safety profile.