Longeveron Proclaims Outcomes of Lomecel-B™ in Phase 2a Clinical Investigation for Treating Alzheimer’s Disease
Longeveron Inc., a biotech firm in a clinical stage that's engineering cell therapies for severe and ongoing aging-related disorders like Alzheimer's disease, hypoplastic left heart syndrome, and Aging-related Frailty, made an optimistic announcement of the preliminary outcomes from the Phase 2a experimentation involving their product under investigation, Lomecel-B™, intended for curing mild cases of Alzheimer’s disease.
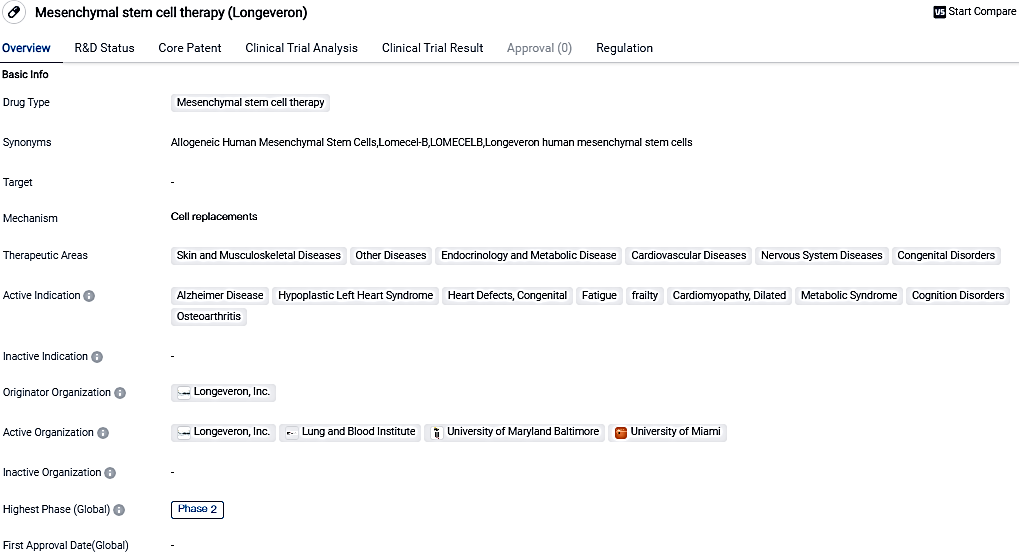
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"We think these findings strongly reinforce the safety and medicinal capacity of Lomecel-B™ in tackling Alzheimer's disease, thereby paving the way for more extensive clinical trials in this area and others," stated Wa’el Hashad, CEO of Longeveron.
"We anticipate divulging more biomarker data from this experiment possibly later this month, which could provide further insights into the medical impacts of Lomecel-B™ on the studied group. We eagerly expect significant progress in the short term and the complete realization of Lomecel-B™'s medicinal potential." Hashad further stated.
"These trials involving Lomecel-B™ show promise," chimed in Dr. Jeffrey Cummings, MD, Vice Chair of Research, UNLV Department of Brain Health. “The trials fulfilled their main safety objective and were backed by the absence of cognitive or atrophy warnings. The effectiveness findings are promising, and these results should lay the groundwork for future research."
"These results, and their apparent validation by the outcomes of Longeveron’s initial phase I Alzheimer’s disease trial, are inspiring," said Dr. Nataliya Agafonova, CMO of Longeveron.
It is estimated that approximately 6.7 million Americans are dealing with Alzheimer's disease. Within the overall U.S. population, approximately 1 in 9 individuals aged 65 and older is affected by Alzheimer's disease. The proportion of people living with Alzheimer's disease rises with age. Even with advances in new anti-amyloid therapy, there is still a significant unfulfilled medical need.
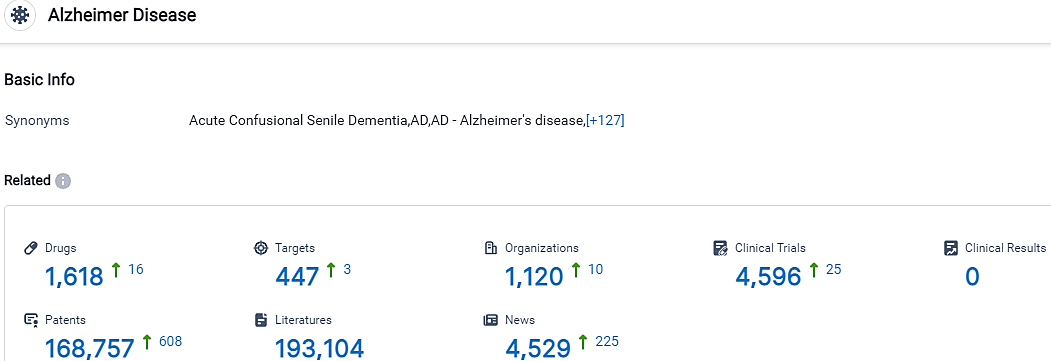
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
According to the data provided by the Synapse Database, As of October 14, 2023, there are 1618 investigational drugs for the Alzheimer’s disease, including 447 targets, 1120 R&D institutions involved, with related clinical trials reaching 4596,and as many as 168757 patents.
Lomecel-B™ is a living cell product made from specialized cells isolated from the bone marrow of young healthy adult donors. Lomecel-B™ may have multiple potential mechanisms of action that may lead to anti-inflammatory, pro-vascular regenerative responses, and therefore may have broad application for a range of rare and aging related diseases.