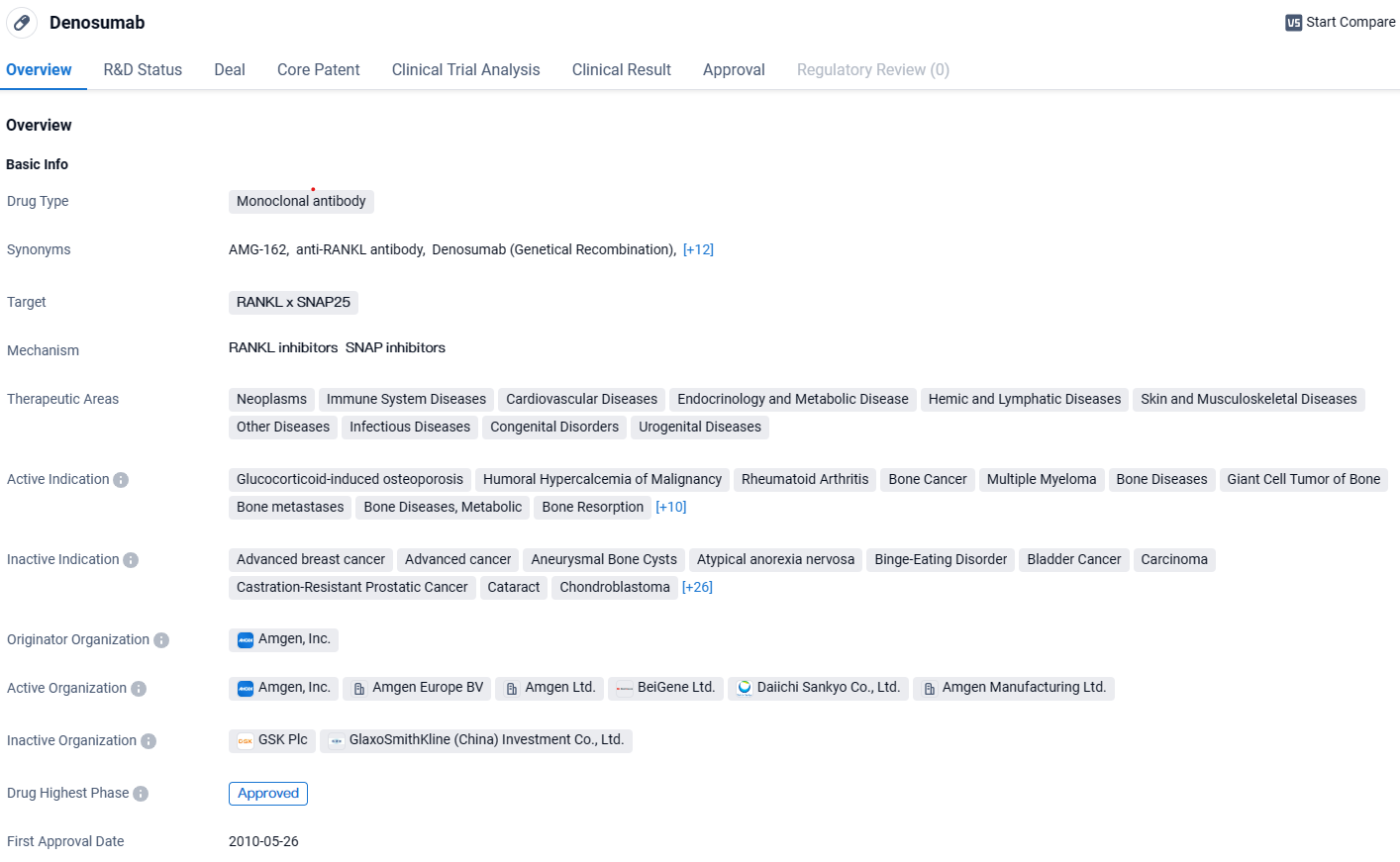
Mabwell's Subsidiary T-mab Gets Approval for Denosumab Injection from National Medical Agency
Mabwell, a pioneering company in the biopharmaceutical field that boasts a comprehensive value chain, has declared that its fully-owned subsidiary, T-mab, has successfully received the green light for its Denosumab Injection from the National Medical Products Administration. Identified in the marketplace under the brand name MAIWEIJIAN and known in research circles by the identifier 9MW0321, this formulation represents the initial biosimilar version of denosumab (with a potency of 120mg) to be sanctioned for commercial distribution within China.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
MAIWEIJIAN is a fully human recombinant monoclonal antibody formulated for injection that targets RANKL. It is sanctioned for use in managing unresectable giant cell tumors within bones or cases where surgery could result in severe functional limitations. This includes its application in both adults and adolescents whose bones have fully developed.
Mabwell disclosed the outcomes of phase 1 and phase 3 trials evaluating the denosumab biosimilar through publications in "International Immunopharmacology" in 2022, and the esteemed "JAMA Oncology" journal in 2024. Detailed comparative studies involving pharmacokinetics and clinical effectiveness in subjects with bone metastases from solid malignancies have thoroughly indicated that 9MW0321 (MAIWEIJIAN) mirrors the reference medicine in terms of pharmacodynamics, clinical outcomes, and safety profile.
Due to its proven efficacy in treatment, denosumab has garnered endorsements from various authoritative consensus statements and medical treatment guidelines. The medicinal community, including prescribers and recipients, acknowledges denosumab's therapeutic benefits with great confidence.
Denosumab presents several benefits as compared to bisphosphonates, which are typically employed in medical treatments for bone-related complications. These advantages include:
1) A mechanism of action that involves direct interference with the RANKL/RANK/OPG signalling pathway, providing effective prevention and management of skeletal complications that result from cancerous bone involvement.
2) Superior clinical efficacy relative to bisphosphonates, including successful outcomes in patients who have not responded to previous bisphosphonate therapy.
3) An advantageous safety profile owing to its non-renal clearance, which results in a reduced risk of kidney-related adverse effects in patients receiving denosumab.
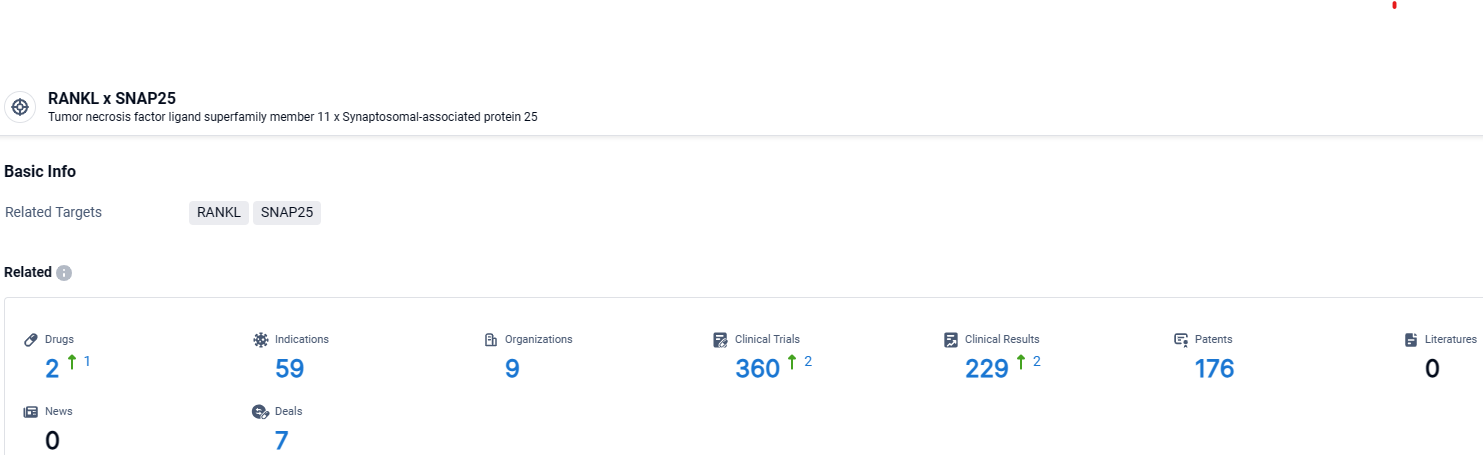
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of April 9, 2024, there are 2 investigational drugs for the RANKL and SNAP25 target, including 59 indications, 9 R&D institutions involved, with related clinical trials reaching 360, and as many as 176 patents.
Denosumab targets RANKL and SNAP25 and has been approved for use in multiple therapeutic areas. Its efficacy in treating various indications, particularly those related to bone health and cancer, has made it a valuable addition to the pharmaceutical industry.