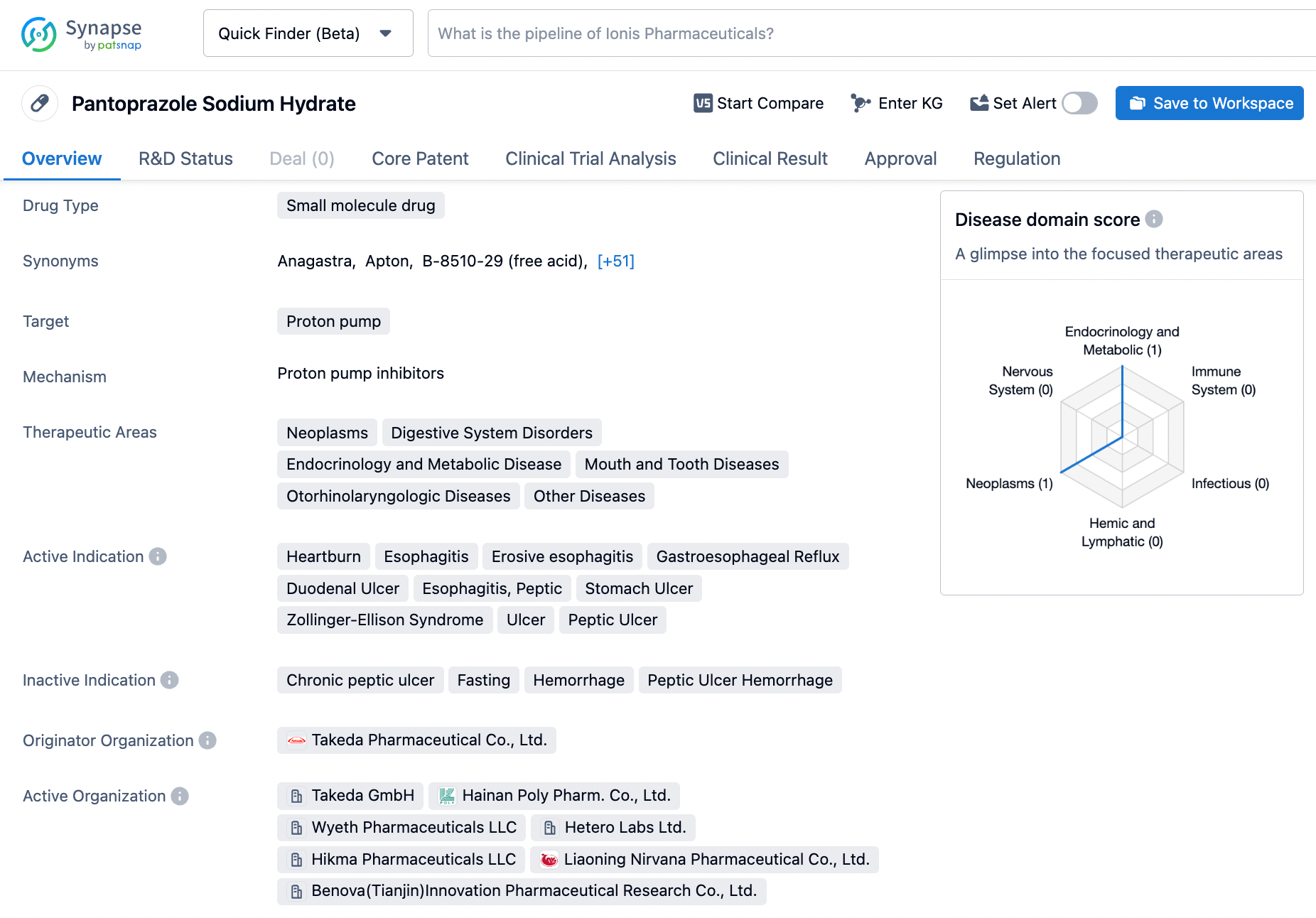
Making the Most Out of Synapse: Searching for Pantoprazole
Pantoprazole is a small molecule medicine classed as a proton pump inhibitor, which means it reduces the amount of acid generated in the stomach. Takeda Pharmaceutical Co., Ltd. authorized this drug for the first time in January 1994. Pantoprazole is exceptionally efficient in treating disorders such as peptic ulcer bleeding, which is characterized by excessive acid output, resulting in stomach lining erosion. It is also used to treat fasting when acid secretion can cause pain and bleeding. Generally speaking, Pantoprazole is well-tolerated and has minimal adverse effects, making it a common therapy choice for acid-related diseases. Click on the image below to begin the exploration journey of Pantoprazole through the Synapse database!
You can search for the latest pharmaceutical information such as drugs, targets, patents, transactions, clinical results, etc. through the Synapse database. Come and experience it!