Marengo Begins Phase 2 Trial of Invikafusp Alfa (STAR0602) in PD-1 Resistant Tumors, Expands Study to Europe
Marengo Therapeutics, Inc., a clinical-stage biotechnology company pioneering novel approaches for precision T cell activation, announced the dosing of the first patient in the Phase 2 portion of its STARt-001 trial. The clinical study builds on the Phase 1/2 trial evaluating invikafusp alfa as a monotherapy in biomarker-enriched patients with advanced anti-PD-1 resistant solid tumors.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The outcomes from the Phase 1 segment of the STARt-001 trial were recently shared in a plenary late-breaking oral session at the SITC Annual Meeting and also in an oral presentation at the ESMO Immuno-Oncology Congress in 2024. These findings confirm the effectiveness of Marengo’s STAR platform design and highlight the early anti-tumor efficacy of invikafusp alfa (STAR0602) as a single agent, showcasing clinical benefits in patients with advanced cancer who have previously been treated extensively and are resistant to anti-PD-1 therapy. Invikafusp alfa displayed a safety profile that is manageable, in line with its innovative mechanism of action, suggesting its promise as a viable treatment for high tumor mutation burden (TMB-H) cancers or virally-linked tumors.
The Phase 2 trial will administer the recommended Phase 2 dose (0.08mg/kg) and is currently enrolling participants in prominent oncology centers across Europe, with initial site activations occurring in France and Spain.
“We are excited to progress invikafusp alfa into Phase 2, collaborating with esteemed European oncology centers,” stated Ke Liu, M.D., Ph.D., Chief Development Officer at Marengo Therapeutics. “The anti-tumor activity observed as a single agent in Phase 1, especially in PD-1-resistant 'cold' tumors like colorectal cancer, reinforces our confidence in this approach and our aspiration to reach a broader patient population. Partnering with notable European institutions not only expands our geographical reach but also enhances our capacity to enroll more patients resistant to PD-1 inhibitors. Our Phase 2 study aims to enhance our comprehension of the mechanism by which invikafusp alfa operates across various tumor types.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
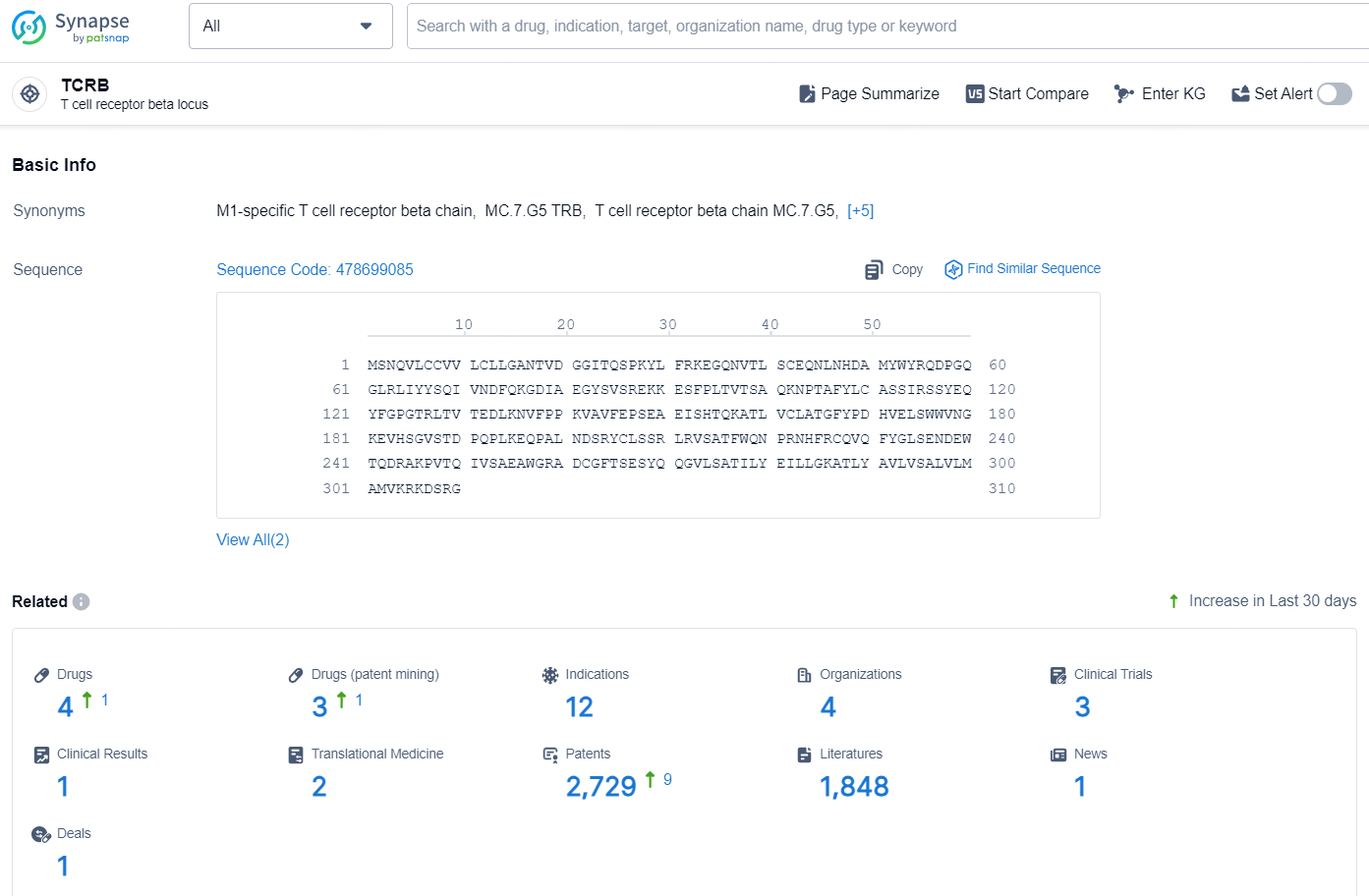
According to the data provided by the Synapse Chemical, As of December 26, 2024, there are 4 investigational drugs for the TCRB target, including 12 indications, 4 R&D institutions involved, with related clinical trials reaching 3, and as many as 2729 patents.
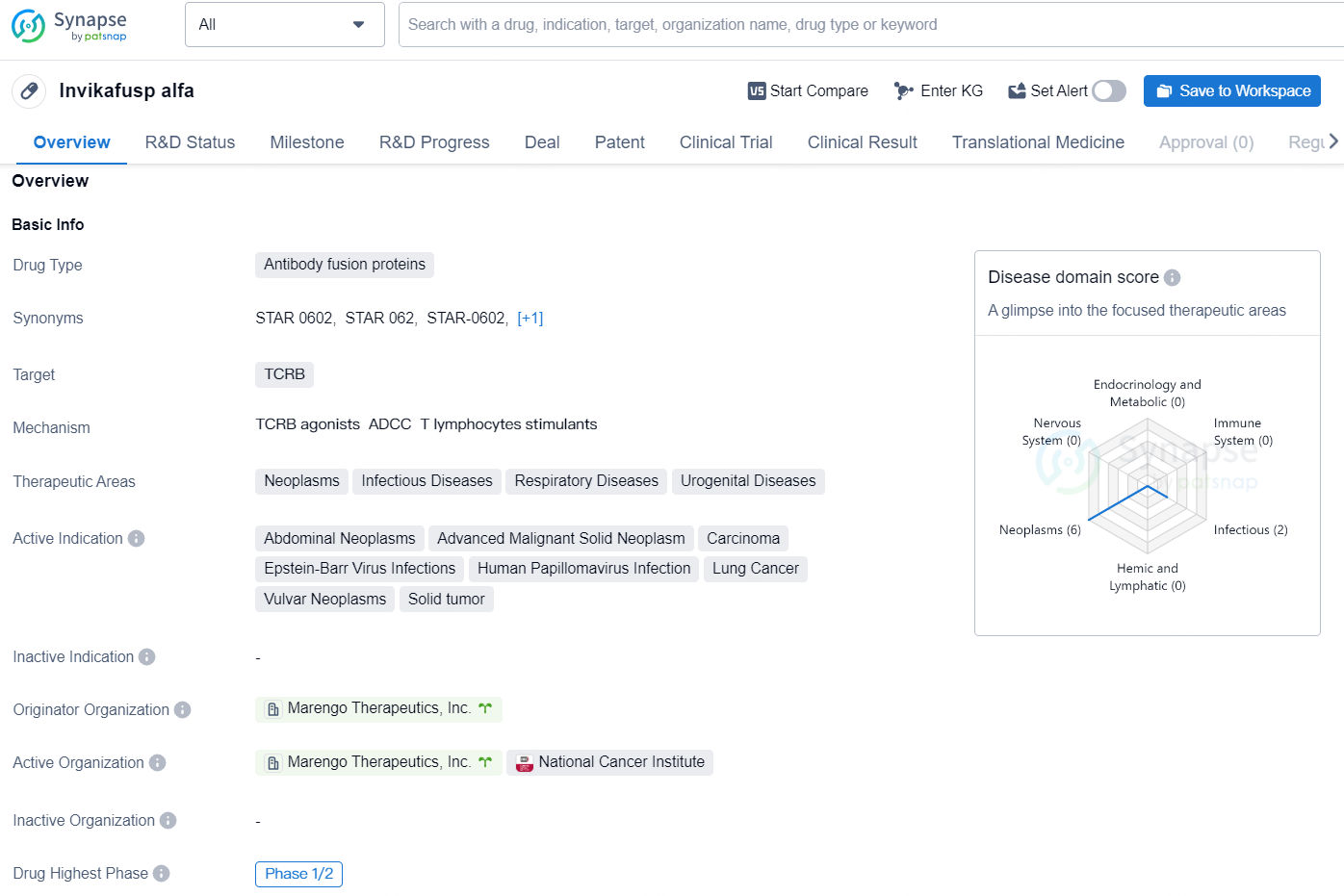
Invikafusp alfa is a drug classified as an antibody fusion protein, and it primarily targets the TCRB. This drug is indicated for various therapeutic areas, including neoplasms, infectious diseases, respiratory diseases, and urogenital diseases. Some of the active indications for Invikafusp alfa include abdominal neoplasms, advanced malignant solid neoplasm, carcinoma, Epstein-Barr virus infections, human papillomavirus infection, lung cancer, vulvar neoplasms, and solid tumor.