Medicenna unveils positive survival results from the Phase 2b trial of Bizaxofusp for recurrent Glioblastoma at the 28th annual NeuroOncology Society meeting
Medicenna Therapeutics Corp., an immunotherapy business currently in the clinical stage, concentrating on the advancement of Superkines, has declared a poster exhibition and a spoken overview featuring extended follow-up outcomes from the Phase 2b medical examination of bizaxofusp, the company's ground-breaking IL-4R focused medication for the cure of recurrent glioblastoma. These will be showcased by Dr. Steven Brem, M.D., at the Society for Neuro-Oncology 2023 Annual Gathering, scheduled from November 15 to 19, 2023, in Vancouver, Canada. The company specializes in the first-to-market segments of healthcare.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"Bizaxofusp presents an innovative technique by showing a dose-responsive effect on the IL-4 receptor, a cytokine known for its immune-suppressive abilities. Additionally, employing bizaxofusp either alone or in combination could possibly 'turn the tables' on glioblastoma, transforming it from an immunologically 'inert' mass into a receptive one, by utilizing convection enhanced delivery to 'take the battle to the tumor'.
This information gives fresh optimism to glioblastoma patients who have sparse selections for treatment upon recurrence," stated Dr. Steven Brem. "The doubling of survival benefit in incurable rGBM resulting from a solitary bizaxofusp treatment, even after an additional 22 months of follow-up, strengthens the reliability of the initial primary analysis," expressed Fahar Merchant, Ph.D.
Medicenna’s President CEO. Dr. Merchant continued, “Working closely with regulatory bodies, our initiatives have secured us a groundbreaking Phase 3 trial design in rGBM using an external control arm and we are actively in search of collaborations to further advance the bizaxofusp registration study.
Following our Phase 2b outcome announcement in the journal Neuro-Oncology® earlier this year, we are excited to share our updated 4-year follow-up findings at SNO 2023 with the medical and research communities.” Medicenna has received approval from the U.S. FDA on the trial design for the Phase 3 LIGHT™ study and is running after potential collaborations to commence the LIGHT trial, and should it be approved, to roll out bizaxofusp across pivotal global markets.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
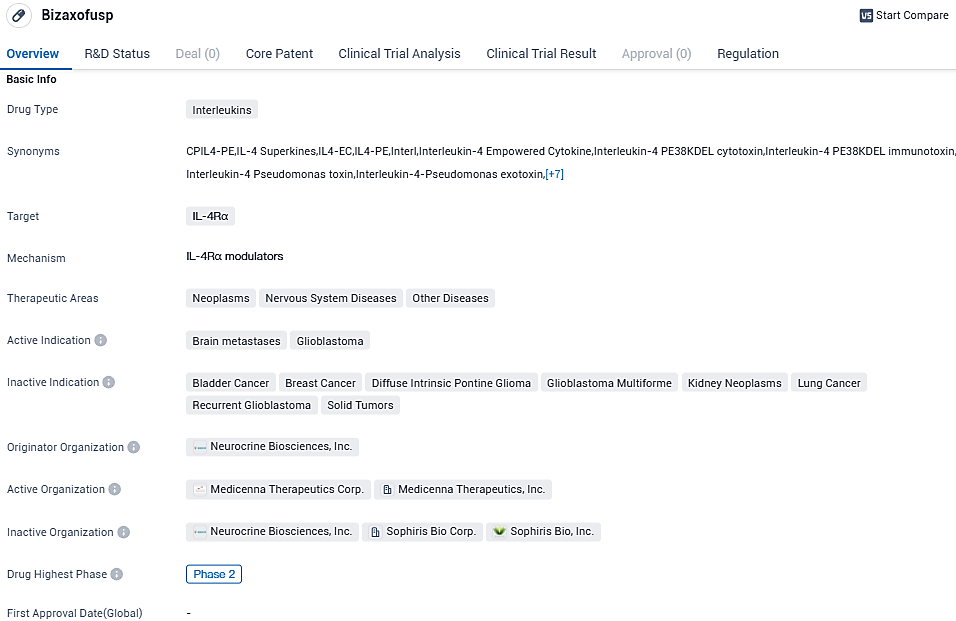
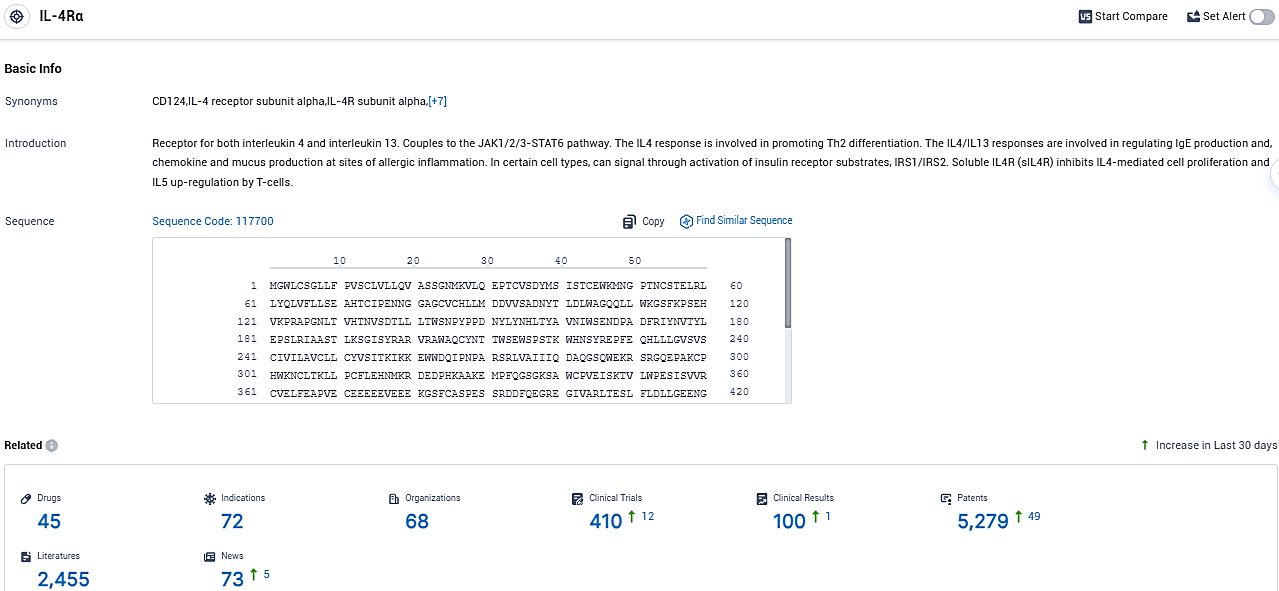
According to the data provided by the Synapse Database, As of November 23, 2023, there are 45 investigational drugs for the IL-4Rα target, including 72 indications, 68 R&D institutions involved, with related clinical trials reaching 410, and as many as 5279 patents.
Bizaxofusp is Medicenna’s IL-4 Empowered Superkine that has been studied in 5 clinical trials in over 130 patients, including a Phase 2b trial in patients with recurrent glioblastoma, the most common and uniformly fatal form of brain cancer. Bizaxofusp has been granted FastTrack and Orphan Drug status from the FDA and FDA/EMA, respectively.