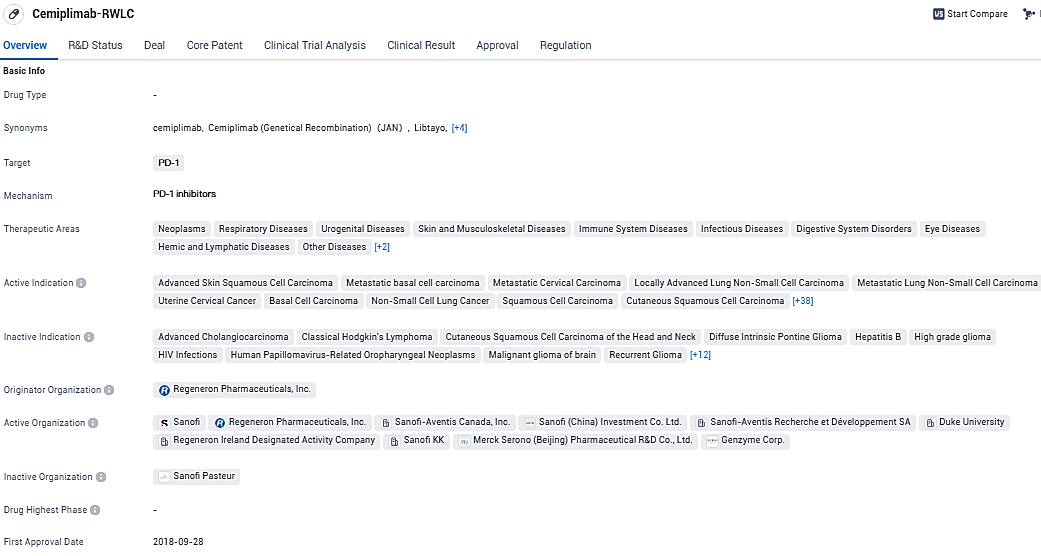
Medison Pharma Strikes Deal to Market Regeneron's Drug Libtayo® (cemiplimab) Across Several Nations
Medison Pharma has disclosed the formation of an exclusive partnership spanning multiple countries with the prominent biotech firm Regeneron Ireland DAC, renowned for its innovative creation and marketing of transformative therapeutic solutions for patients battling severe illnesses. Under this alliance, Medison Pharma has secured the rights to distribute Libtayo® (cemiplimab), an entirely human-derived monoclonal antibody that interacts with the PD-1 immune checkpoint receptor found on T cells. This agreement encompasses selected European territories as well as various other global regions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The pharmaceutical Libtayo was developed within the research facilities of Regeneron using their exclusive VelocImmune® technology. Regeneron acquired the complete and exclusive rights for the global development, commercialization, and production of Libtayo from Sanofi in the month of July 2022. During the initial six months of the year 2024, Medison will collaborate with Regeneron to ensure that the handover of the commercial operations is smooth and well-coordinated, working closely with regulatory bodies and all involved parties.
Libtayo is authorized for use by the health authorities in upwards of 25 countries, including clearance from the European Medicines Agency. This innovative therapy is at the forefront of its class as a PD-1 inhibitor and has become the standard treatment option for particular types of non-melanoma skin cancers.
For specific patients suffering from advanced stages of basal cell carcinoma and those diagnosed with advanced cutaneous squamous cell carcinoma, Libtayo is available as a solo therapy. In certain cases, it is also authorized as either a standalone treatment or paired with chemotherapy for advanced non-small cell lung cancer, as well as for single-agent therapy in recurring or widely spread cervical cancer in distinct markets.
Meir Jakobsohn, the Founder and Executive Chairman at Medison remarked, "Our extensive commercial network is perfectly suited for Regeneron's strategic market entry, providing another excellent chance for Medison to improve access to groundbreaking treatments aimed at rare and serious conditions." The decision by Regeneron to partner with Medison is a testament to the remarkable benefits that our cross-regional approach can deliver for leading biotech firms focusing on niche markets."
Adding to the excitement, Gil Gurfinkel, the Chief Executive Officer at Medison, expressed, "We are honored to forge a partnership with Regeneron. Our unique, centralized commercial infrastructure stands out by providing a consolidated management solution across various territories and regions, presenting immense advantages to an increasing number of elite biotech organizations. Our system is strategically designed to convert multiple complex and segmented markets into a singular, cohesive commercial territory."
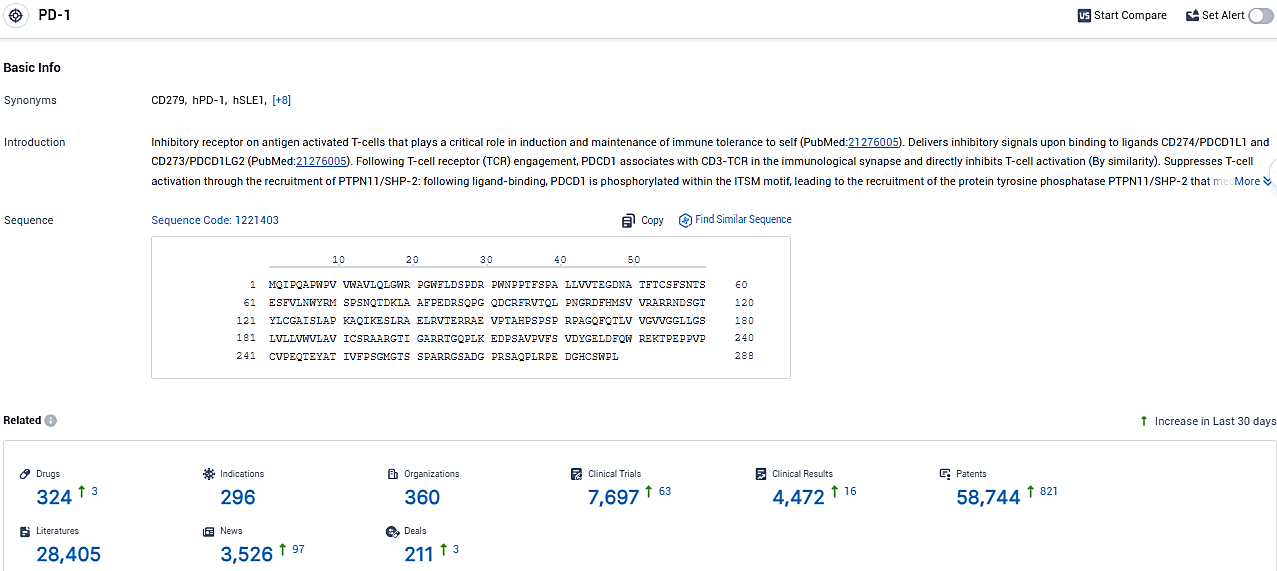
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of January 13, 2024, there are 324 investigational drugs for the PD-1 tagets, including 296 indications, 360 R&D institutions involved, with related clinical trials reaching 7697, and as many as 58744 patents.
Libtayo is a fully human monoclonal antibody targeting the immune checkpoint receptor PD-1 on T cells and was invented using Regeneron's proprietary VelocImmune® technology. As of July 1, 2022, Libtayo is developed and marketed globally by Regeneron. Outside of the U.S., the generic name for Libtayo is cemiplimab.