Advances in Clinical Research on NMDA Receptor Antagonist
The N-Methyl-D-Aspartate (NMDA) receptor is a type of ionotropic glutamate receptor that plays a crucial role in excitatory synaptic transmission, plasticity, and excitotoxicity in the central nervous system, closely related to the body's memory, learning, and emotions.
As a type of neurotransmitter, glutamate receptors play a vital role in neuronal signal transmission. Their expression quantity, distribution, and type all affect normal neurophysiological functions. Reversing the functional changes in these receptors has important implications for the treatment or prevention of neurological diseases.
NMDA Receptor Competitive Landscape
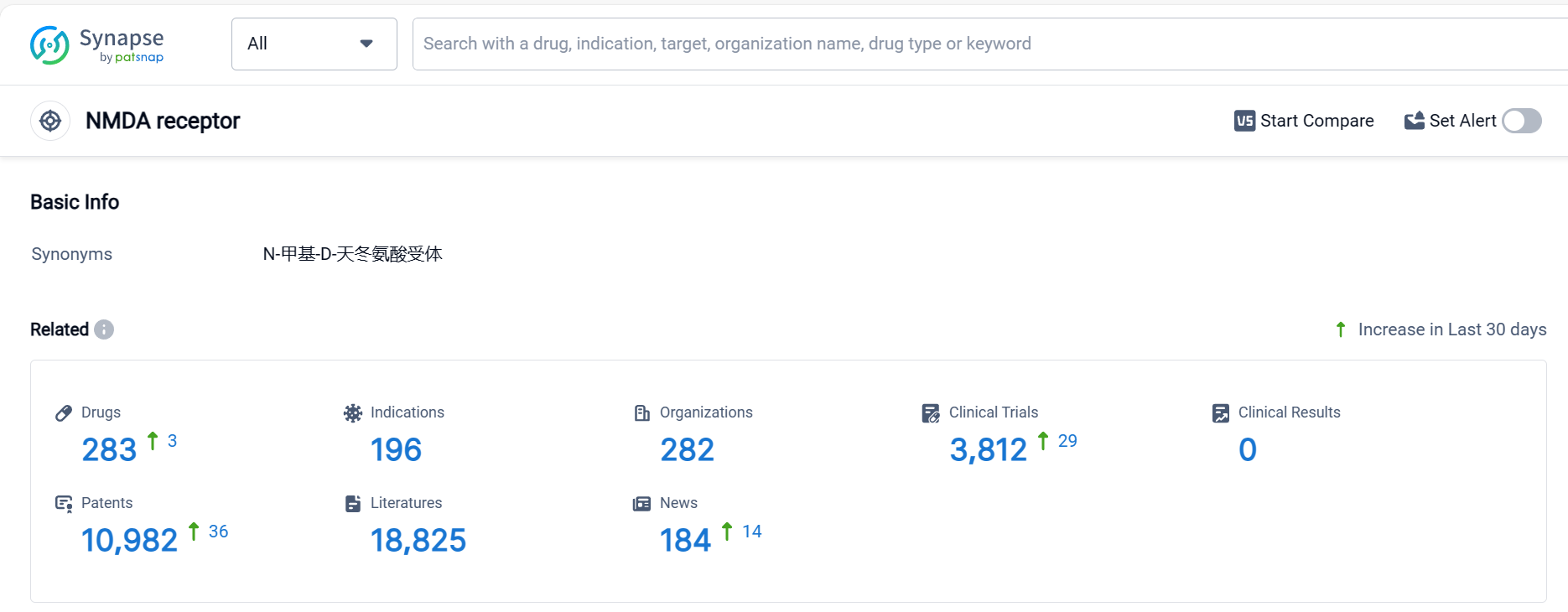
According to Patsnap Synapse, as of 5 Oct 2023, there are a total of 283 NMDA receptor drugs worldwide, from 282 organizations, covering 196 indications, and conducting 3812 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
The analysis of the target NMDA receptor reveals a competitive landscape with multiple companies actively developing drugs. The highest stage of development is the "Approved" phase, indicating successful drug development in various indications. Small molecule drugs are progressing most rapidly, with a significant number of approved drugs. China is emerging as a key player in the development of drugs targeting the NMDA receptor. Further analysis of R&D progress, biosimilars, and specific indications is required to provide more detailed insights into the current competitive landscape and future development of the target NMDA receptor.
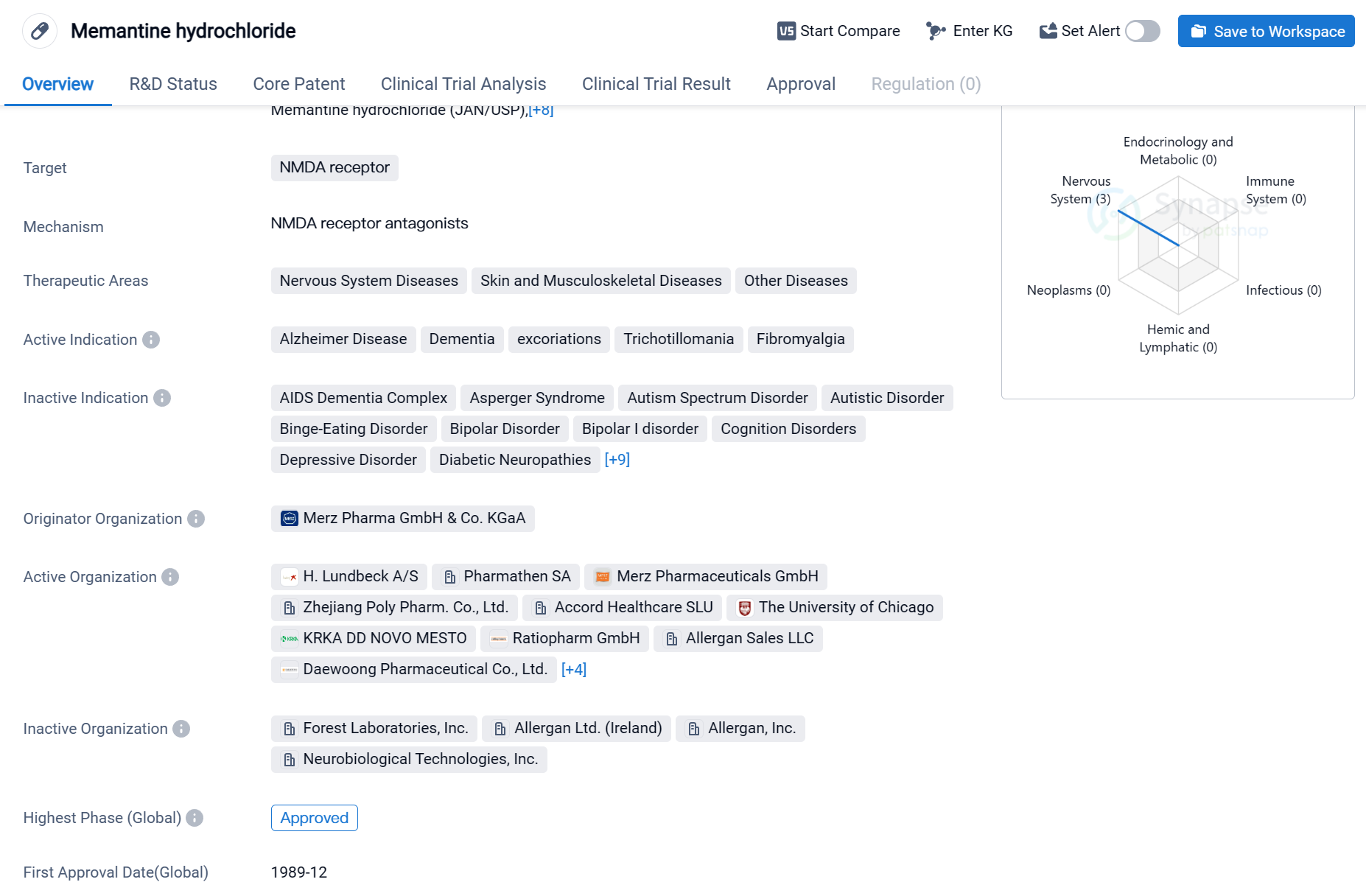
Key drug:Memantine hydrochloride
Memantine hydrochloride is a small molecule drug that targets the NMDA receptor. It is primarily used in the treatment of nervous system diseases, skin and musculoskeletal diseases, and other diseases. The drug has been approved for the treatment of Alzheimer's disease, dementia, excoriations, trichotillomania, and fibromyalgia.
The originator organization of Memantine hydrochloride is Merz Pharma GmbH & Co. KGaA. The drug has reached the highest phase of approval globally. It was first approved for use in 1989 in Germany.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Memantine hydrochloride is primarily used in the treatment of Alzheimer's disease, a progressive neurodegenerative disorder that affects memory, thinking, and behavior. It works by blocking the activity of the NMDA receptor, which is involved in the transmission of signals between nerve cells in the brain. By blocking this receptor, Memantine hydrochloride helps to regulate the activity of glutamate, a neurotransmitter that is believed to play a role in the development of Alzheimer's disease.
In addition to Alzheimer's disease, Memantine hydrochloride has also been approved for the treatment of dementia, a general term for a decline in mental ability severe enough to interfere with daily life. It is also used in the treatment of excoriations, a condition characterized by the repetitive picking or scratching of the skin, trichotillomania, a disorder that involves the recurrent pulling out of one's hair, and fibromyalgia, a chronic pain disorder.
The approval of Memantine hydrochloride in multiple therapeutic areas highlights its potential in addressing various diseases affecting the nervous system, skin, and musculoskeletal system. Its approval in both global and Chinese markets further emphasizes its significance and potential impact on patient care.
Overall, Memantine hydrochloride is a small molecule drug that targets the NMDA receptor and has been approved for the treatment of Alzheimer's disease, dementia, excoriations, trichotillomania, and fibromyalgia. Its originator organization is Merz Pharma GmbH & Co. KGaA, and it has reached the highest phase of approval globally and in China. The drug's approval in 1989 in Germany marks its first approval for use.