Merus Reports Initial Dosing in Phase 3 LiGeR-HN2 Trial of Petosemtamab for 2/3L r/m HNSCC
Merus N.V., a clinical-stage oncology company focused on developing groundbreaking full-length multispecific antibodies, has announced the enrollment of the first patient in its phase 3 trial. This trial aims to assess the efficacy and safety of petosemtamab, a Biclonics targeting EGFR and LGR5, in comparison to the investigator's choice of single-agent chemotherapy or cetuximab in patients who have previously been treated for recurrent or metastatic head and neck squamous cell carcinoma. This study is known as the LiGeR-HN2 trial.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Merus has received feedback from the U.S. Food and Drug Administration (FDA) indicating that a dosage of 1500 mg of petosemtamab administered biweekly is suitable for further development in HNSCC, both as a standalone treatment and in combination with pembrolizumab.
"With the promising clinical results of petosemtamab in HNSCC and confirmation from the FDA on the dosage, we are thrilled to have commenced treating our first patient in the phase 3 trial for the 2nd/3rd line," stated Fabian Zohren, M.D., Ph.D., Chief Medical Officer at Merus. "We are confident that petosemtamab could establish a new standard of care for r/m HNSCC."
Known also as MCLA-158, petosemtamab is a Biclonics low-fucose human full-length IgG1 antibody that targets both the epidermal growth factor receptor (EGFR) and the leucine-rich repeat-containing G-protein-coupled receptor 5 (LGR5).
Petosemtamab is engineered to have three distinct mechanisms of action: it inhibits EGFR-dependent signaling, promotes EGFR internalization and degradation in cancer cells by binding to LGR5, and enhances antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP).®
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
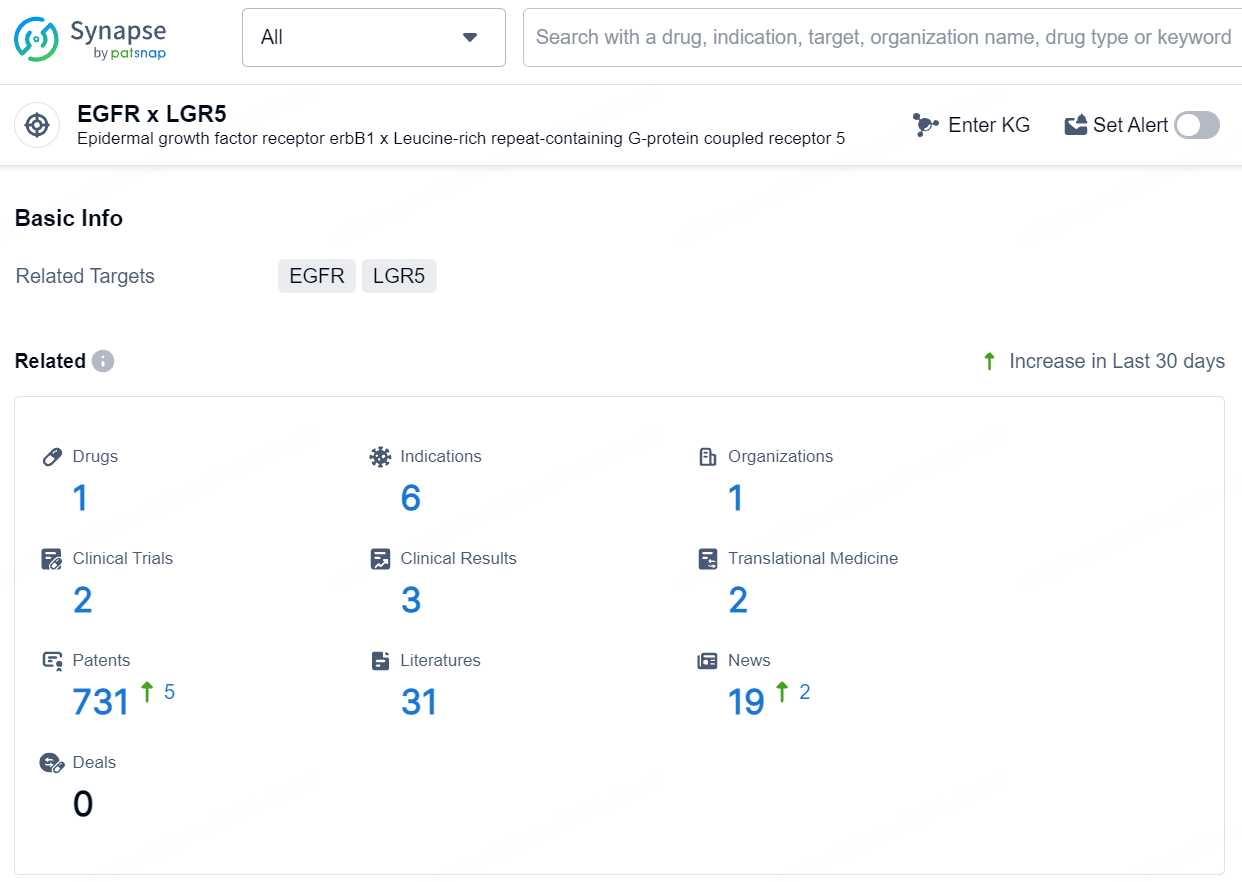
According to the data provided by the Synapse Database, As of July 30, 2024, there are 1 investigational drug for the EGFR and LGR5 targets, including 6 indications, 1 R&D institution involved, with related clinical trials reaching 2, and as many as 731 patents.
The highest phase of development for Petosemtamab is Phase 3, indicating advanced research and potential for regulatory approval in the near future. In terms of regulatory status, the drug has been granted Fast Track and Breakthrough Therapy designations, highlighting its potential for significant therapeutic advancement in the treatment of the aforementioned indications.