Is Ublituximab approved by the FDA?
Ublituximab, marketed under the brand name Briumvi, is a CD20 monoclonal antibody used in the treatment of multiple sclerosis (MS). Ublituximab was approved by the FDA on December 28, 2022. Specifically, it targets relapsing forms of MS, including clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease.
Uses for Ublituximab
Ublituximab is utilized in treating adults with relapsing forms of MS. This includes:
- Clinically isolated syndrome
- Relapsing-remitting MS
- Active secondary progressive MS
These forms of MS are characterized by periods of new or increasing neurological symptoms (relapses) followed by periods of partial or complete recovery (remissions).
Administration and Dosage
Ublituximab is administered intravenously by a healthcare provider. The dosing regimen is as follows:
- First Infusion: 150 mg IV over at least 4 hours.
- Initiate at a rate of 10 mL per hour for the initial 30 minutes.
- Increase to 20 mL per hour for the next 30 minutes.
- Increase to 35 mL per hour for the next hour.
- Increase to 100 mL per hour for the remaining 2 hours.
- Second Infusion (2 weeks after the first): 450 mg IV over at least 1 hour.
- Initiate at a rate of 100 mL per hour for the first 30 minutes.
- Increase to 400 mL per hour for the remaining 30 minutes.
- Subsequent Infusions (every 24 weeks): 450 mg IV over at least 1 hour.
- Initiate at a rate of 100 mL per hour for the first 30 minutes.
- Increase to 400 mL per hour for the remaining 30 minutes.
Premedication with corticosteroids, antihistamines, and antipyretics is recommended to reduce the frequency and severity of infusion reactions.
Precautions and Side Effects
Precautions:
- Allergies: Inform your doctor about any allergies, including those to medications, foods, dyes, preservatives, or animals.
- Hepatitis B: If you have had hepatitis B, it may reactivate or worsen. Regular liver function tests are required during and after treatment.
- Infections: Patients with active infections or chronic infections should inform their healthcare provider.
- Pregnancy: Ublituximab can harm an unborn baby. Effective birth control is required during treatment and for at least 6 months after the last dose.
- Breastfeeding: Consult your doctor to understand the potential risks.
Side Effects:
Common side effects of ublituximab include:
- Dizziness
- Nausea
- Light-headedness
- Itching
- Sweating
- Headache
- Chest tightness
- Back pain
- Trouble breathing
- Swelling in the face
More serious side effects include:
- Allergic reactions (hives, difficulty breathing, swelling of face, lips, tongue, or throat)
- Brain infections that can lead to disability or death
- Symptoms of infection (fever, chills, sore throat, body aches, unusual tiredness, loss of appetite, bruising, or bleeding)
Conclusion
Ublituximab (Briumvi) is an FDA-approved treatment for relapsing forms of multiple sclerosis, offering a new option for patients battling this challenging disease. Approved on December 28, 2022, it has shown promise in managing the symptoms and progression of MS. Patients should consult their healthcare providers to understand the potential benefits and risks associated with ublituximab therapy.
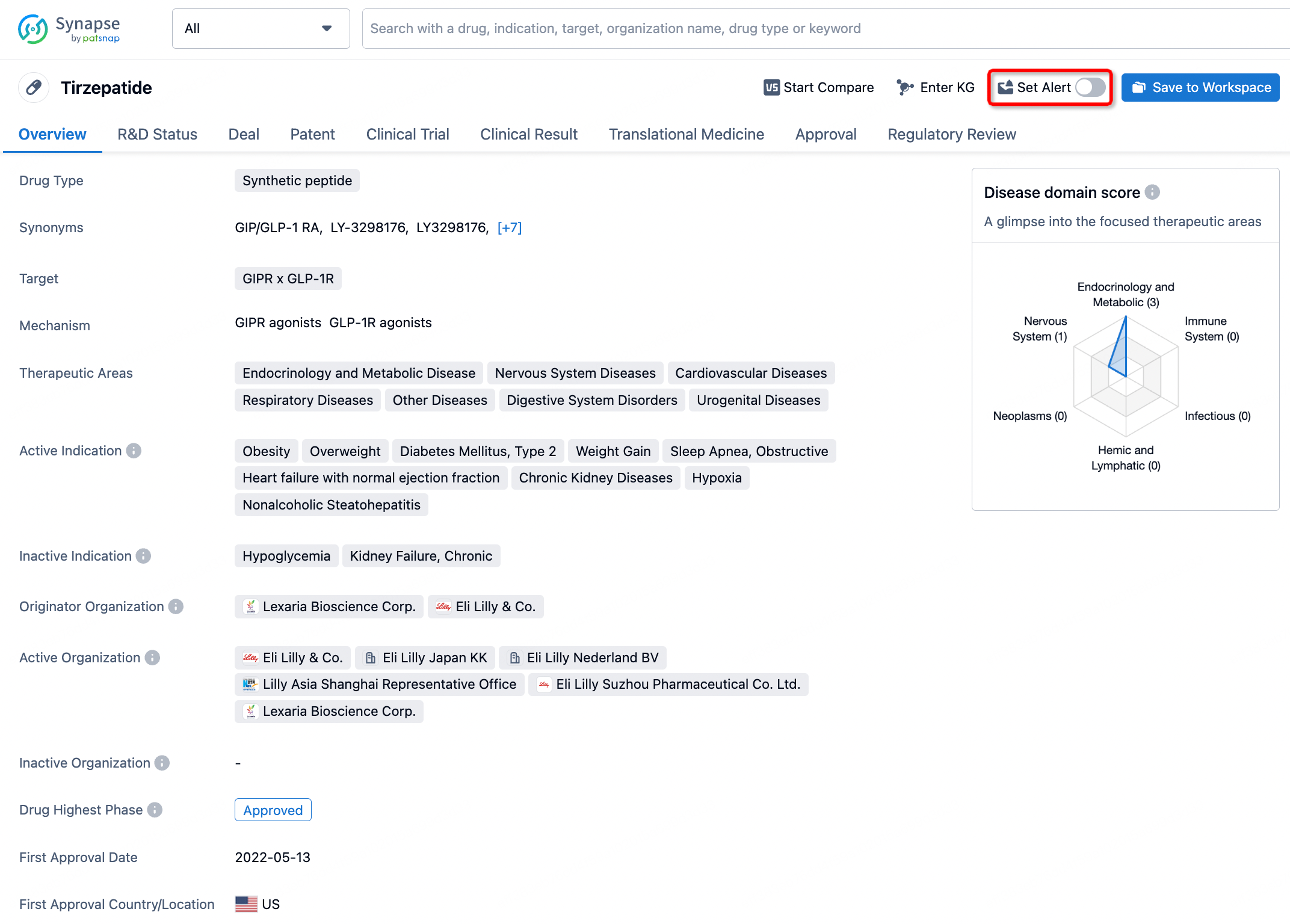
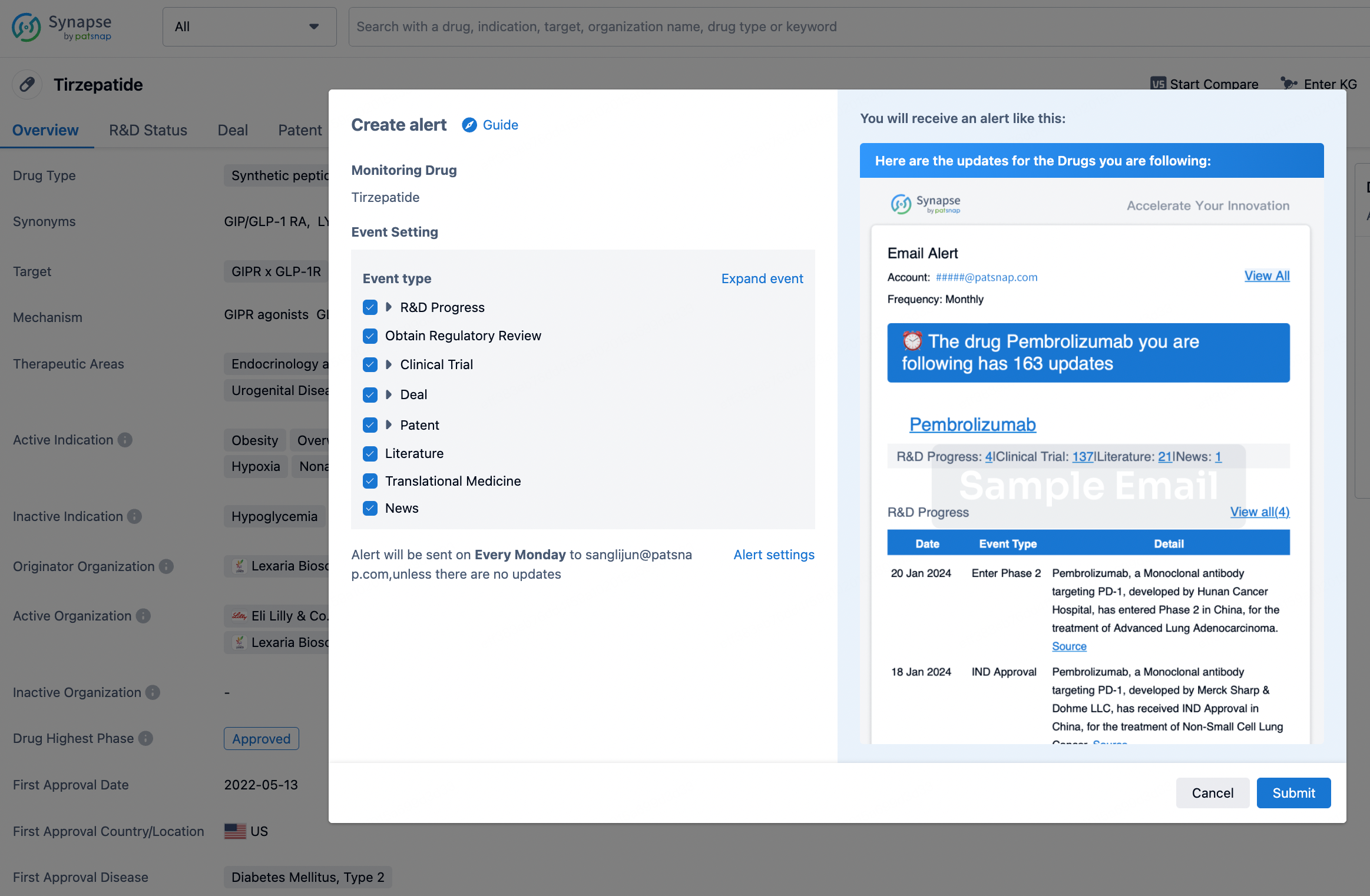
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!