Is Lecanemab approved by the FDA?
Lecanemab, marketed under the brand name Leqembi, was granted accelerated approval by the FDA on January 6, 2023. This approval was based on the observed reduction in amyloid beta plaques in patients treated with the medication. The continued approval for this indication is contingent upon verification of clinical benefits in a confirmatory trial.
Uses for Lecanemab
Lecanemab is specifically used to treat adults with Alzheimer's disease. It works by targeting and reducing amyloid beta plaques in the brain, which are thought to play a central role in the pathophysiology of Alzheimer's disease.
Administration and Dosage
Lecanemab is administered via an intravenous infusion by a healthcare provider. The typical dosing regimen is an infusion of 10 mg/kg given over approximately one hour, once every two weeks. Before starting treatment, the presence of amyloid beta pathology should be confirmed through appropriate diagnostic methods.
Precautions and Side Effects
Warnings:
- Lecanemab can cause a condition known as Amyloid Related Imaging Abnormalities (ARIA), which involves temporary swelling or small spots of bleeding in the brain. This condition usually resolves over time but can be serious. Symptoms may include headaches, dizziness, nausea, confusion, trouble with walking, seizures, or vision changes.
- Lecanemab can cause temporary swelling or bleeding in the brain, which may not always present symptoms but can be detected through MRI scans.
Precautions:
- Pregnancy and Breastfeeding: The effects of lecanemab on an unborn baby are not fully known. Patients should inform their doctor if they are pregnant or planning to become pregnant. It is also important to discuss with a doctor whether it is safe to breastfeed while using lecanemab.
- Interactions: Patients should inform their doctor about all other medications they are taking, especially those used to treat or prevent blood clots, including aspirin.
Common Side Effects:
- ARIA with symptoms or signs that appear on an MRI
- Infusion-related reactions
- Headache
Patients should seek emergency medical help if they experience signs of an allergic reaction such as hives, difficulty breathing, or swelling of the face, lips, tongue, or throat. Other side effects that may occur during the injection include dizziness, nausea, light-headedness, itching, sweating, headache, flu-like symptoms, fever, vomiting, chest tightness, fast/slow heartbeats, back pain, trouble breathing, or swelling in the face.
Conclusion
Lecanemab (Leqembi) is an FDA-approved medication for the treatment of Alzheimer's disease, granted accelerated approval on January 6, 2023. It offers a promising treatment option by targeting amyloid beta plaques in the brain. Patients should consult their healthcare providers to understand the potential benefits and risks associated with lecanemab therapy.
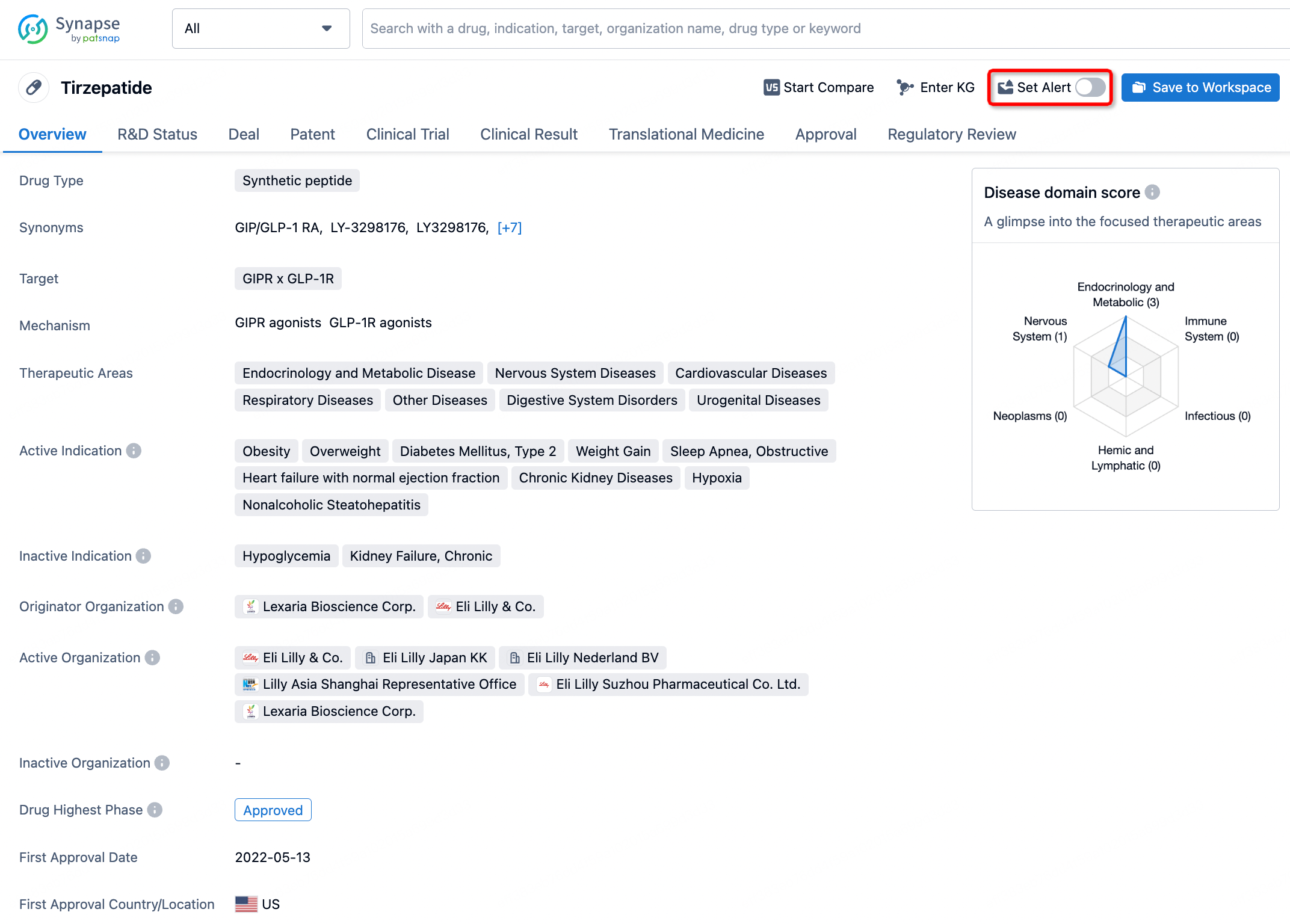
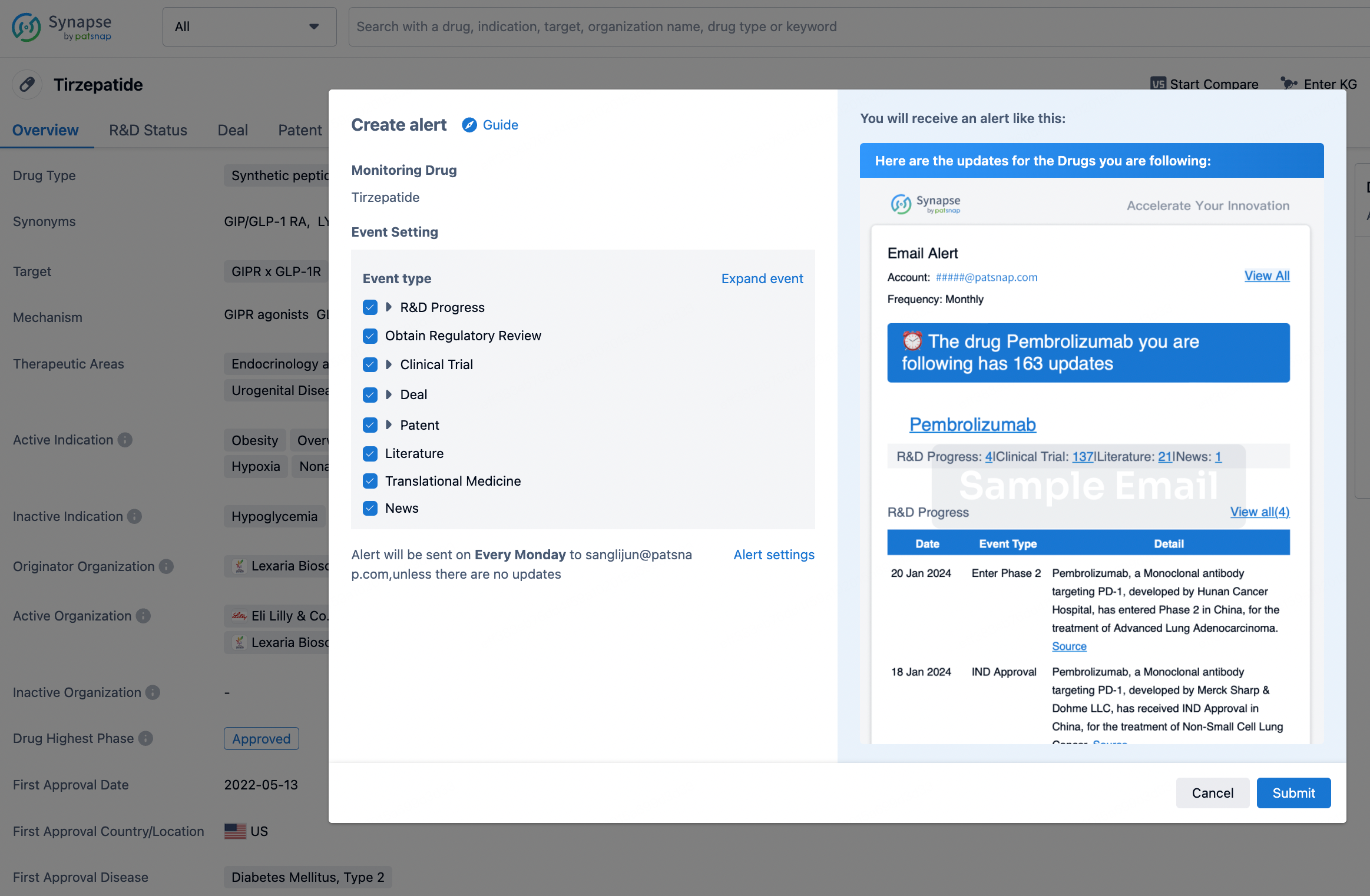
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!