Mesentech Begins Administering Experimental Medicine MES1022 to Speed Up Bone Fracture Recovery in Initial Patients
Mesentech, a biotechnology firm dedicated to creating innovative, tissue-selective treatments for various bone diseases, recently revealed that its Phase 1 trial has begun, and the initial patients have already received their doses of MES1022. This drug is designed specifically for bone regeneration, with its effectiveness bolstered by its ability to be selectively distributed to bone tissues, thereby minimizing systemic exposure and potential side effects.
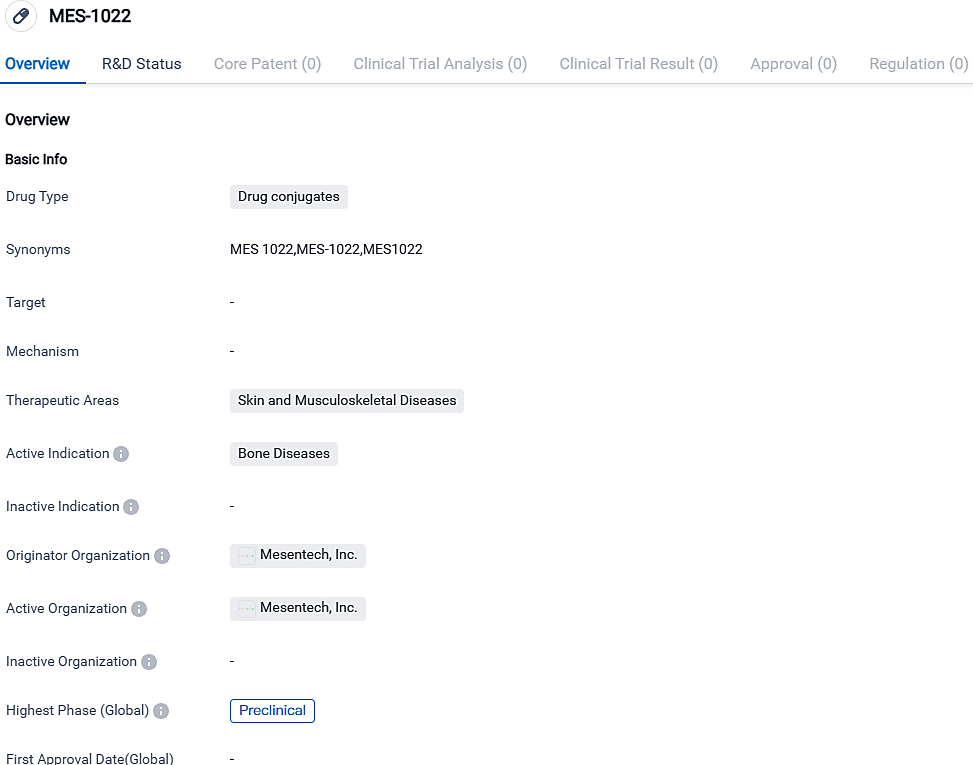
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The optimization of drug delivery to bones continues to be a significant hurdle in devising remedies for bone illnesses. The bone, a complicated, dynamic tissue with distinct characteristics, presents a considerable barrier to efficient drug distribution. Mesentech's specialized prodrug technology solves this problem by facilitating direct drug delivery to the bone.
The firm triumphantly administered the first few doses to patients through underneath-the-skin injections in a Phase 1 trial studying MES1022. This is a bone-specific EP4 receptor agonist prodrug under development to encourage bone regrowth. The initial use for this would be the acceleration of bone fracture healing. An additional scheme utilizing tissue-aiming technology is in progress to treat bone cancers like osteosarcoma and bone metastases.
MES1022 is a prodrug that singularly disperses to bones and delivers a potent prostaglandin imitation through a sustained release system. It has been proven that MES1022 encourages bone regrowth and secondary muscle growth after administering once every week on a systemic level.
“Currently, MES1022 has the potential to become the first non-biological drug predominantly marketed to orthopedic surgeons,” stated Jonathan Polak, CEO of Mesentech.
“With sustained research and development, our specialized prodrugs have potential to revolutionize treatments of previously unapproachable bone disorders by delivering medications precisely to the bone thereby improving safety and efficacy.”
"Recently, even the most technologically advanced medical instruments and implant procedures do not tackle the fundamental biological aspects needed for bone healing," stated David Dvorak, Chairman of the Mesentech board of directors and former President and CEO of Zimmer Biomet. "This innovative treatment might potentially allow orthopedic surgeons to save their patients from distressing and costly repeat surgeries, with decreased risks and extended recovery durations."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
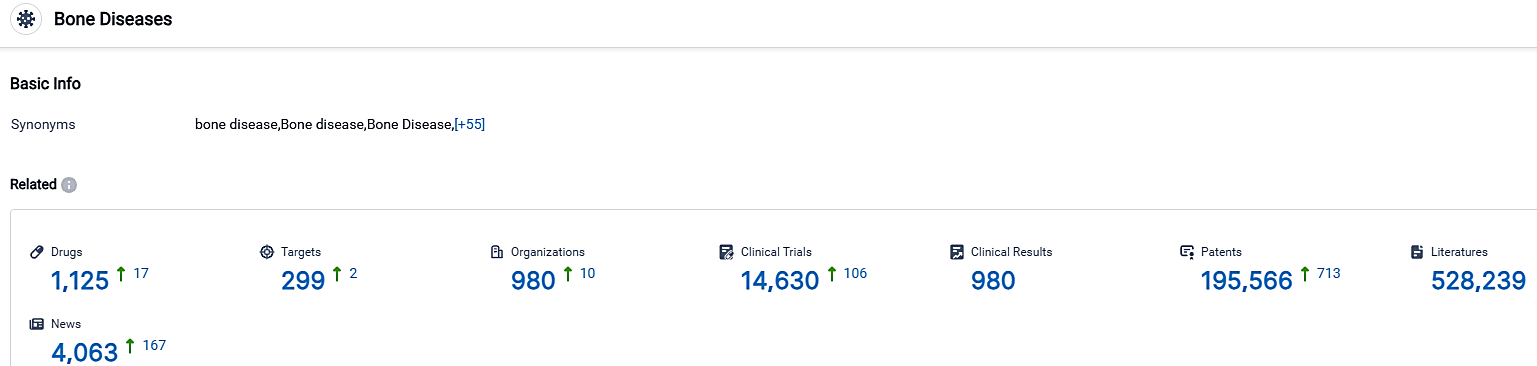
According to the data provided by the Synapse Database, As of October 2, 2023, there are 1125 investigational drugs for the Bone Diseases, including 299 targets, 980 R&D institutions involved, with related clinical trials reaching 14630,and as many as 195566 patents.
Mesentech, Inc. is in the process of developing a drug conjugate known as MES-1022 for the management of bone illnesses. The objective of MES-1022, being a drug conjugate, is to offer tailored treatment by merging a curative component with a specific targeting molecule. Nonetheless, more investigations and medical trials are necessary to evaluate the drug's effectiveness and human safety.