Moderna Unveils Phase 1/2 Results for mRNA-1083
Moderna, Inc. reported encouraging preliminary outcomes from the mRNA-1083 Phase 1/2 study, a combination vaccine currently under investigation for its effect against both influenza and COVID-19. Moderna's experimental combination vaccines aim to benefit individuals, medical professionals, and healthcare networks by promoting better adherence, simplifying the process of administration, and creating more convenience.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
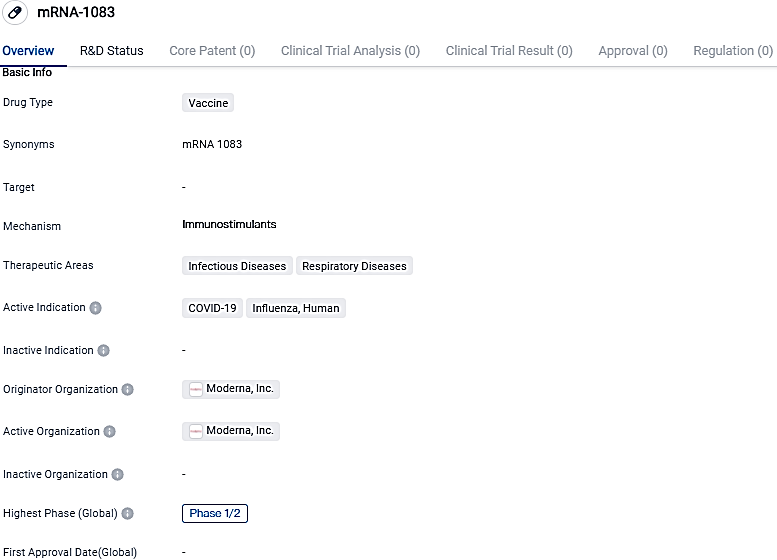
An ongoing Phase 1/2 clinical study, which is randomized and observer blind, is currently evaluating the immunogenicity and safety of mRNA-1083 in contrast to a standard dose of the influenza vaccine, Fluarix, in subjects aged 50 to 64, and an enhanced flu vaccine, Fluzone HD, in individuals aged between 65 to 79. Both these age groups were also assessed in comparison to the Spikevax booster using mRNA-1083.
The mRNA-1083 candidate that was chosen for progression to Phase 3 demonstrated haemagglutination inhibition antibody titers equivalent or better than the two approved quadrivalent influenza vaccines, and showed SARS-CoV-2 neutralizing antibody titers akin to the Spikevax bivalent booster during the Phase 1/2 investigation. In adults aged between 50 to 64 years, mRNA-1083 generated geometric mean titer ratios greater than 1.0 when compared to Fluarix, for all four strains of the influenza vaccine.
In individuals aged between 65 to 79 years, the GMT ratios for mRNA-1083 in comparison to Fluzone HD were also over 1.0, for all four variants of the influenza vaccine. When compared to Spikevax bivalent, GMT ratios of mRNA-1083 were more than 0.9 in those aged 50 to 64 years, and over 1.0 for those in the 65 to 79 years age bracket.
Influenza epidemics occur seasonally, with the level of severity varying each year. The disease causes respiratory issues and places substantial pressure on health services. Despite the existence of various flu vaccines, influenza annually results in severe illnesses in 3-5 million people, and between 290,000 to 650,000 respiratory fatalities associated with influenza. Influenza can affect all age groups but it hits older individuals harder.
Approximately 500-600 million doses make up the global influenza market annually, with around 150 million doses given out in the United States. Moderna forecasts the U.S. COVID-19 market size for fall 2023 to be between 50 to 100 million doses, subject to vaccination rates. In time, the firm anticipates the COVID-19 market will align with the influenza market within the U.S. due to the impact of the disease.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
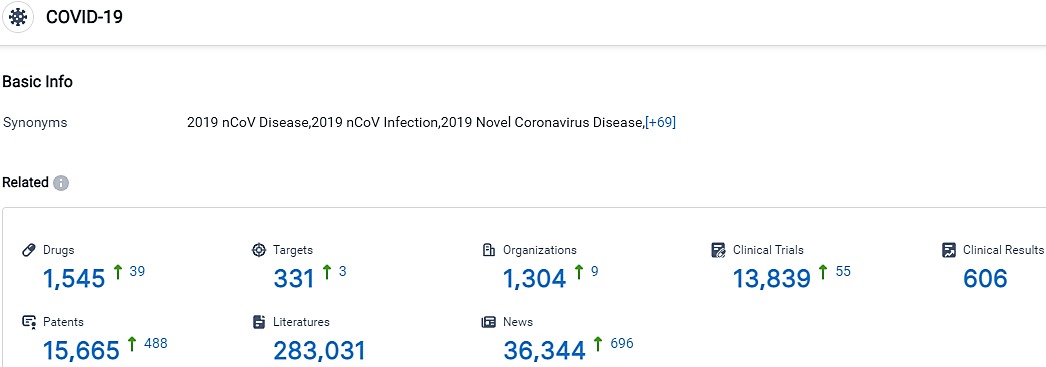
According to the data provided by the Synapse Database, As of October 14, 2023, there are 1545 investigational drugs for the COVID-19, including 331 targets, 1304 R&D institutions involved, with related clinical trials reaching 13839,and as many as 15665 patents.
mRNA-1083 is a vaccine being developed for the prevention of COVID-19 and Influenza in humans. It utilizes mRNA technology to stimulate an immune response. Currently, the drug is in Phase 1/2 of its global development, with further testing required to determine its efficacy and safety.