uniQure Begins Phase I/IIa Trial of AMT-191 for Fabry Disease with Initial Patient Dosed
uniQure N.V. (NASDAQ: QURE), a top gene therapy firm dedicated to developing breakthrough treatments for patients with critical health conditions, has reported that the inaugural patient has received a dose in a Phase I/IIa clinical trial of AMT-191 targeting Fabry disease, an uncommon hereditary genetic disorder. This Phase I/IIa investigation is a multi-site, open-label study underway in the United States, involving two dose-escalation groups to evaluate the safety, tolerability, and preliminary indicators of efficacy of AMT-191 in patients afflicted by Fabry disease.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"We are delighted to commence patient dosing for AMT-191 in Fabry disease, which signifies a major landmark in our objective to progress three new gene therapy candidates into clinical trials this year,” remarked Walid Abi-Saab, M.D., chief medical officer of uniQure. “AMT-191 employs the same AAV delivery technology used in HEMGENIX®, known for its long-term safety profile and proven efficacy in patients with preexisting neutralizing antibodies to the AAV capsid. Our study aims to measure established endpoints in Fabry disease and swiftly generate clinical proof-of-concept data for AMT-191, showcasing a distinctive product profile compared to other Fabry programs currently in clinical trials.”
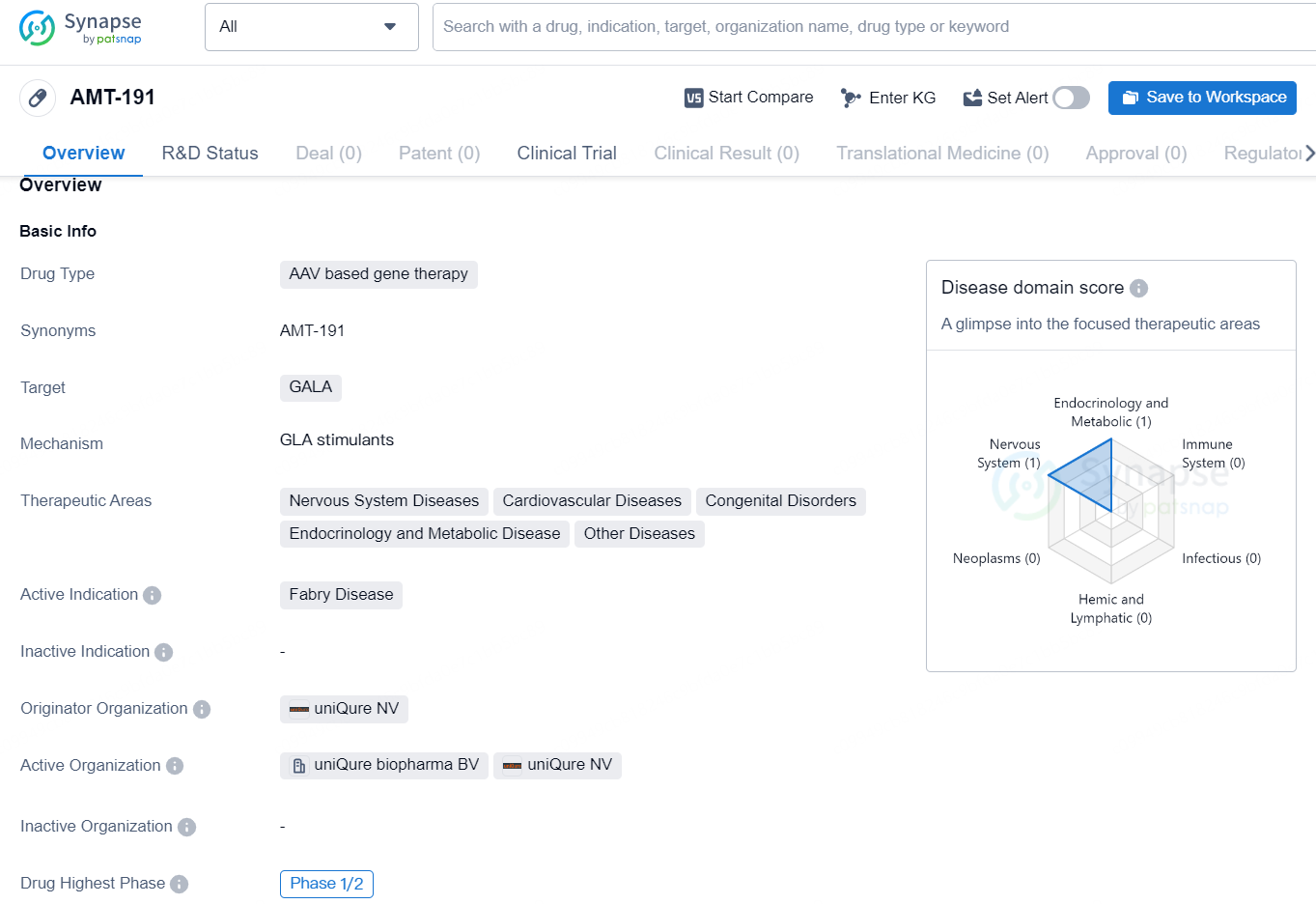
AMT-191 is an investigational gene therapy based on AAV5, utilizing a highly potent promoter to deliver a galactosidase alpha (GLA) transgene specifically targeting the liver to produce the GLA protein. In individuals with Fabry disease, a pathogenic variant in the GLA gene results in α-galactosidase A (aGAL-A) enzyme deficiency, causing a buildup of lipids in various cell types, including those in the kidneys and heart, leading to a multi-system disorder. AMT-191 might provide a unique, one-time intravenous treatment option for Fabry disease.
The AMT-191 Phase I/IIa clinical trial will take place in the United States. This multicenter, open-label trial comprises two cohorts of up to six adult male participants each: a lower-dose cohort receiving 6×10^13 gc/kg and a higher-dose cohort receiving 3×10^14 gc/kg through a single intravenous infusion. Participants will continue their current enzyme replacement therapy until the withdrawal criteria are met and will be monitored for 24 months. The trial will assess safety, tolerability, and early indications of efficacy by measuring the expression of the lysosomal enzyme aGLA-A. Further details can be found on www.clinicaltrials.gov (NCT06270316).
“This accomplishment signifies an exciting phase for our company as we advance additional programs into clinical trials this year,” commented Matt Kapusta, chief executive officer of uniQure. “Building on our momentum, we are concentrating on numerous catalysts within our pipeline, including engaging with the FDA to expedite the clinical pathway for AMT-130 in Huntington’s disease, and initiating new clinical studies in temporal lobe epilepsy and SOD1-ALS. With a robust financial position and extended runway through the end of 2027 due to various cost reduction initiatives, we believe we are well-positioned to achieve key value-generating milestones.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
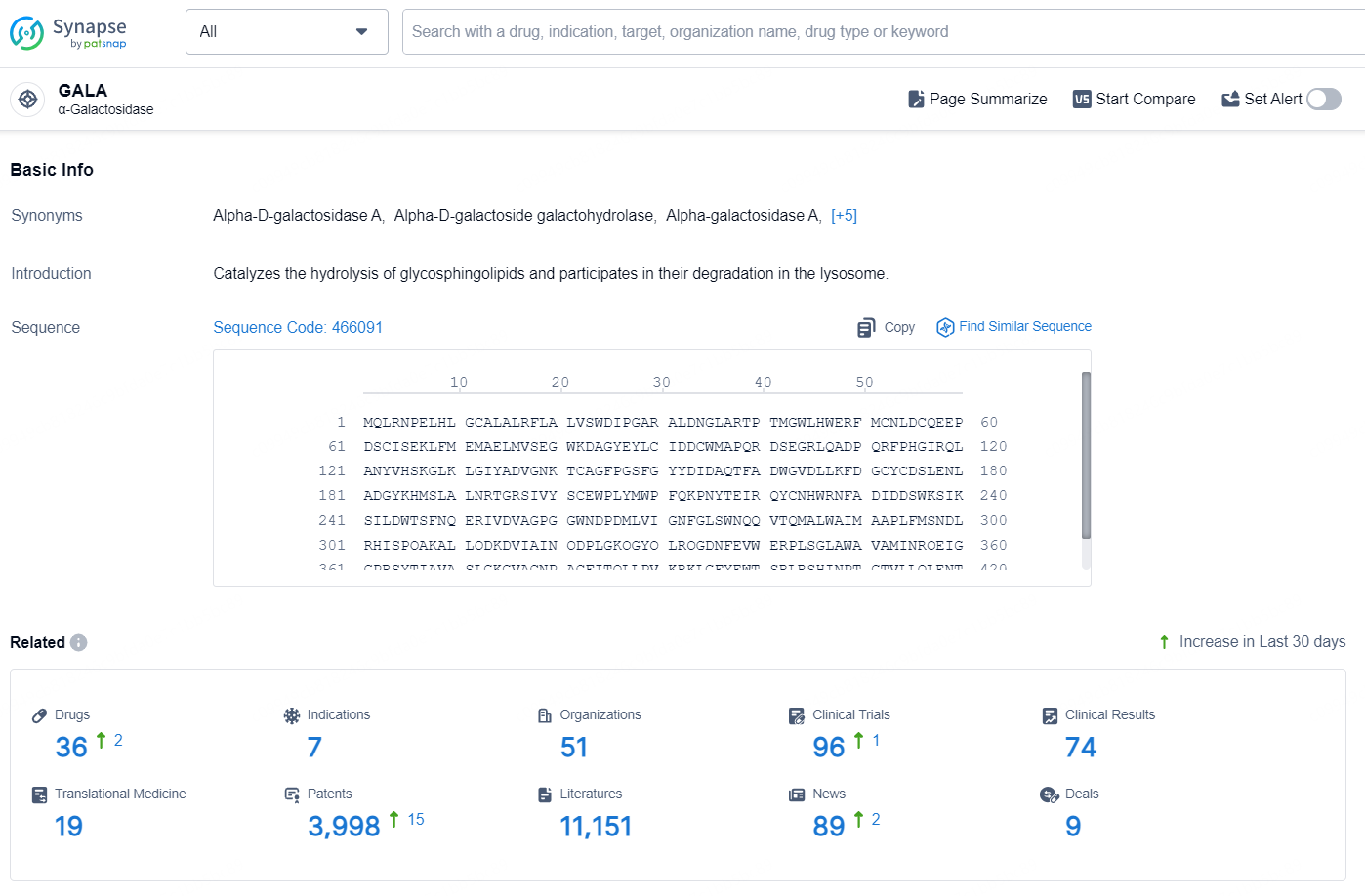
According to the data provided by the Synapse Database, As of August 20, 2024, there are 36 investigational drugs for the GALA targets, including 7 indications, 51 R&D institutions involved, with related clinical trials reaching 96, and as many as 3998 patents.
AMT-191 is an AAV-based gene therapy drug developed by uniQure NV. It targets the GALA gene and is intended to treat Fabry disease, a rare genetic disorder that can affect various systems in the body. The drug has shown potential to address not only Fabry disease but also other therapeutic areas including nervous system diseases, cardiovascular diseases, congenital disorders, endocrinology and metabolic disease, and other diseases.