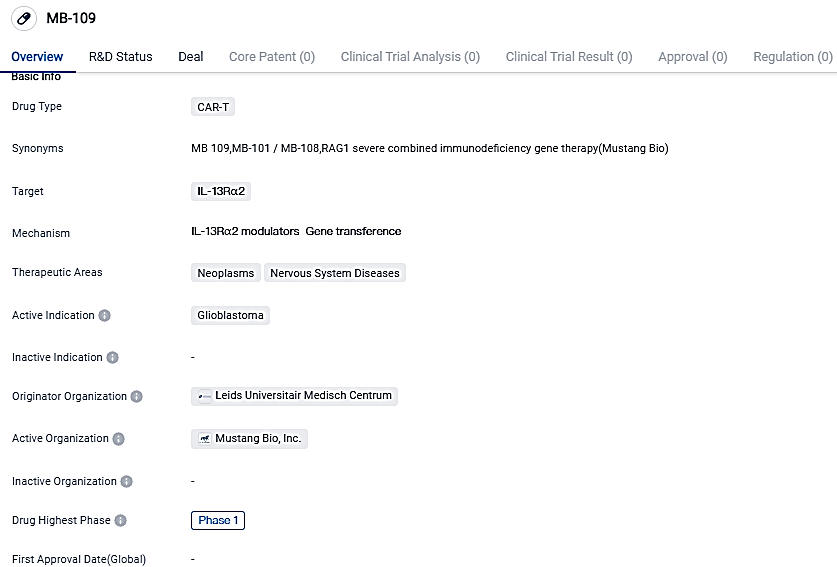
Mustang Bio confirms that FDA has approved the IND application for MB-109, aimed at treating recurrent Glioblastoma and High-Grade Astrocytoma
Mustang Bio, Inc., a bio-pharmaceutical corporation involved in clinical trials and is dedicated to transforming medical advancements in cell and gene therapies into potential remedies for challenging cancers and unusual genetic ailments, has communicated that the FDA has agreed to their IND application of MB-109 intended for managing recurrent glioblastoma and high-grade astrocytoma.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mustang is set to commence a Phase 1 multicenter clinical investigation at City of Hope and the University of Alabama at Birmingham, assessing the safety, efficacy, and tolerability of MB-109, a groundbreaking blend of MB-101 and MB-108. It will be administered to adult patients suffering from recurring GBM and high-grade astrocytomas, wherein IL13Rα2 is expressed on the tumor cells' surface.
Previously shared preclinical data, exhibited at the American Association for Cancer Research Annual Meeting in 2022, backed this combined therapy as a possible way to augment outcomes in treating recurrent GBM. This combination takes advantage of MB-108 to modify the tumor microenvironment and convert cold tumors to “hot,” potentially enhancing the effectiveness of MB-101 CAR-T cell therapy.
Distinct data on MB-101 and MB-108 indicated that the introduction of these treatments was well accepted in patients with recurrent GBM. Notably, out of the 53 Phase 1 patients from COH revealed at AACR in 2022, 2 complete responses were noticed in the 2 patients whose tumors were the “hottest” before being treated with MB-101. Phase 1 clinical investigations of MB-101 at COH and MB-108 at UAB are still in progress.
Mustang's President and CEO, Manuel Litchman, M.D., expressed his satisfaction with the FDA's approval of their IND application for MB-109. He added that FDA's approval within a month of the initial application, regardless of the innovative nature of the combination therapy and the complexity of the trial design, is a testament to the ability and ingenuity of their team.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
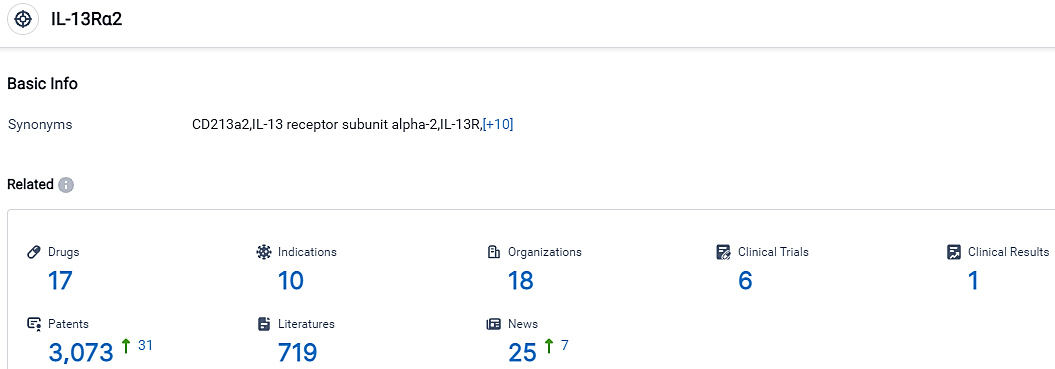
According to the data provided by the Synapse Database, As of October 30, 2023, there are 17 investigational drugs for the IL-13Rα2 target, including 10 indications, 18 R&D institutions involved, with related clinical trials reaching 6,and as many as 3073 patents.
The therapy plan combined with MB-101 and MB-108 is referred to as MB-109 by Mustang. This combo is formulated to utilize MB-108 in turning cold tumors “hot”, thereby potentially enhancing the effectiveness of MB-101 CAR-T cell therapy. MB-109 holds the possibility to meet the unmet medical need in the treatment of glioblastoma, and it could be applied more widely to neoplasms and nervous system disorders.