Toripalimab: FDA Greenlights First made-in-China PD-1 and Its Evolving Competitive Landscape
With the FDA's approval of toripalimab monoclonal antibody for use in all treatment regimens for recurrent or metastatic nasopharyngeal carcinoma (NPC), the first Chinese PD-1 is set to enter the U.S. market.
Junshi Biosciences became the first Chinese manufacturer to receive approval for a checkpoint inhibitor in China in 2018 and is the drug’s original developer. However, Coherus BioSciences pre-paid $150 million at the start of 2021 to secure the rights in the U.S. and Canada. The antibody is marketed as Tuoyi in China and under the brand name Loqtorzi in the U.S., with a planned launch in the first quarter of 2024.
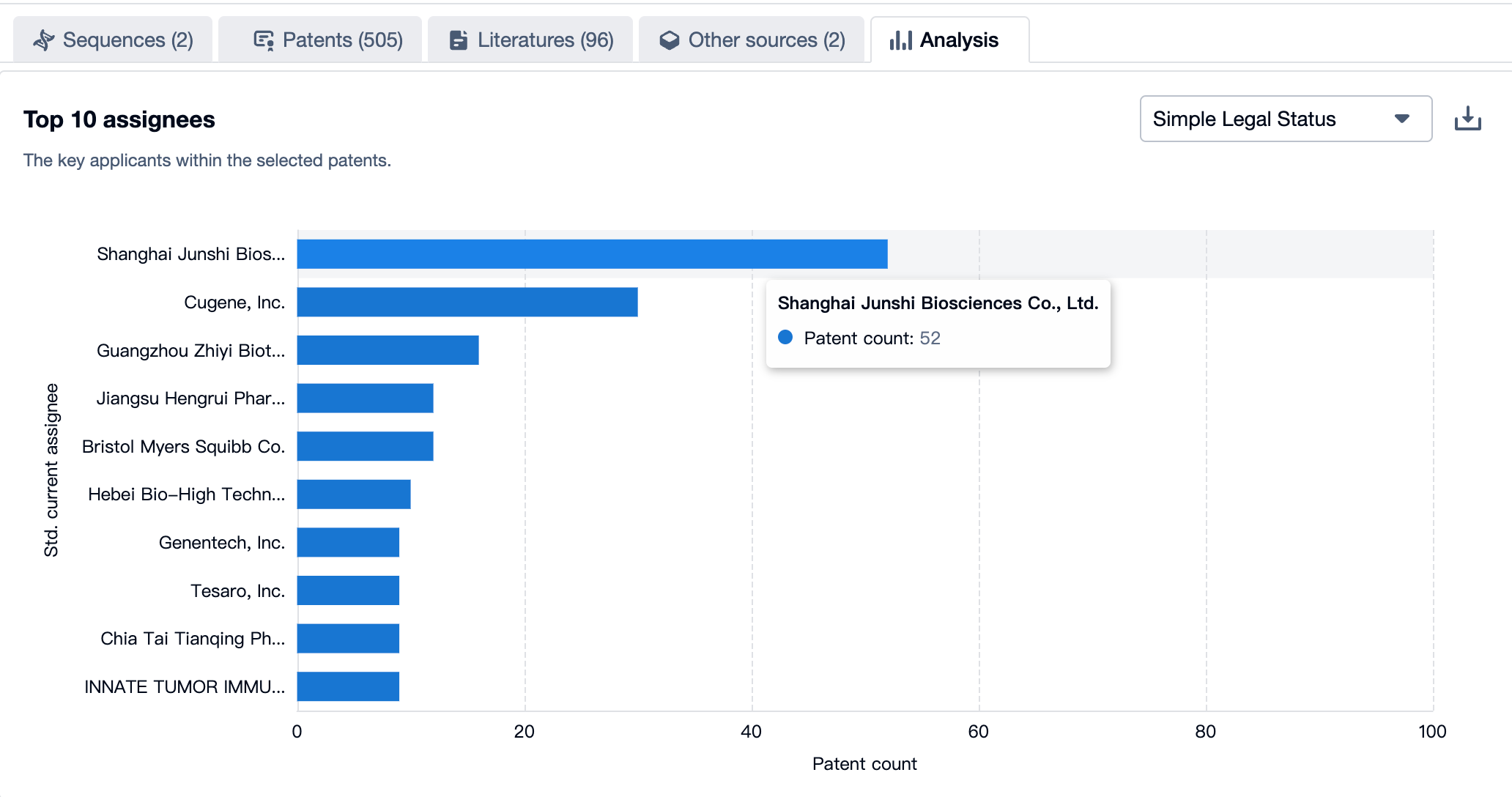
A Panoramic View of Toripalimab’s Top 10 Patent Assignees
According to the Patsnap Bio Sequence Database, the top 10 applicants for the toripalimab patent are Shanghai Junshi Biosciences with 52 applications, Cugene with 30 applications, and Guangzhou Zhiyi Biotech with 16 applications. For more applicants, please refer to the image below.
Source, Patsnap Bio
Evolution of the Layout of Top 10 Patent Assignees over the years, the Competitive landscape over time
Exploring the changes occurring within the application structure amongst toripalimab's top 10 patent assignees over time delivers insights into the competitive landscape's evolution.
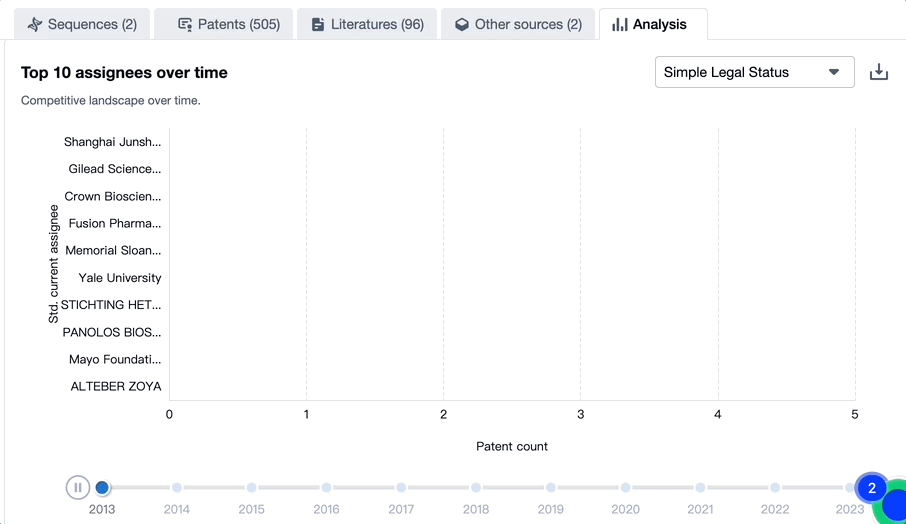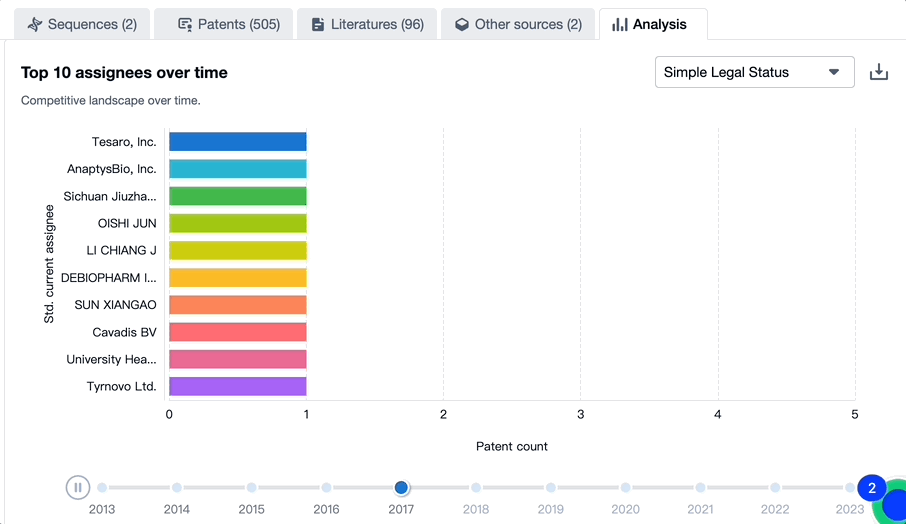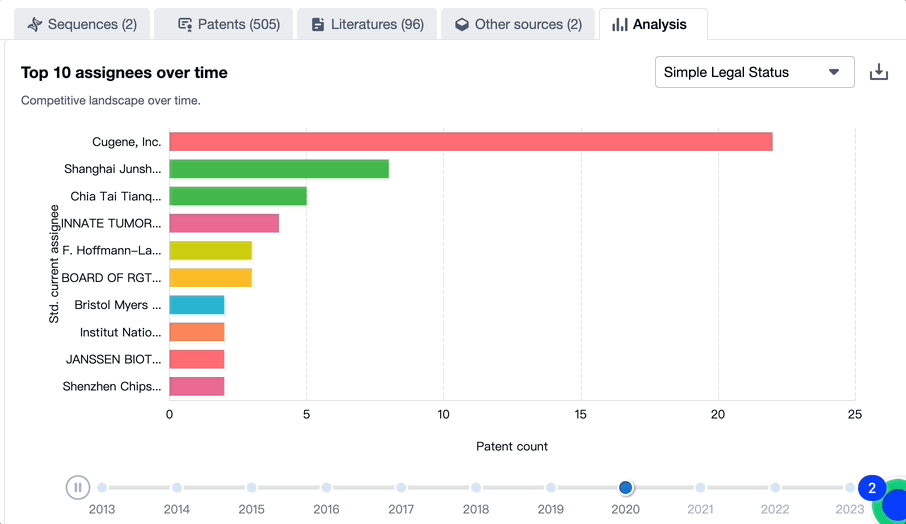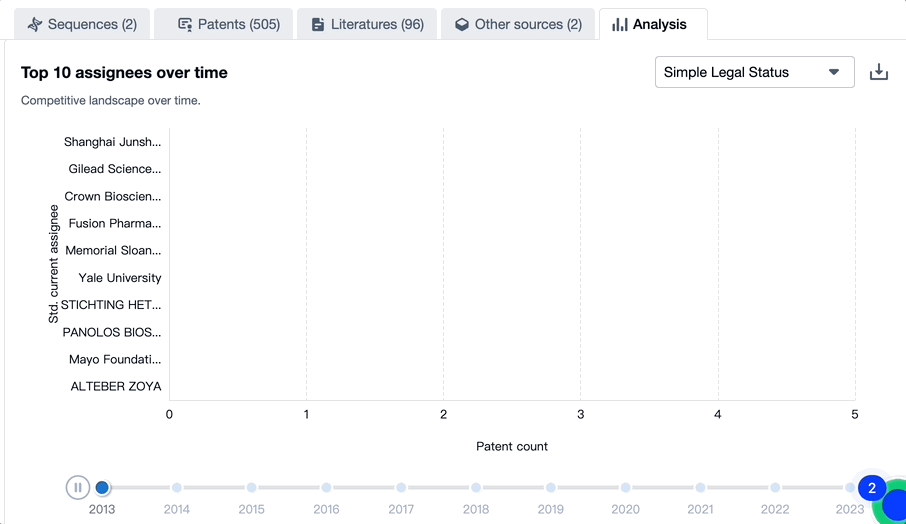
Source, Patsnap Bio
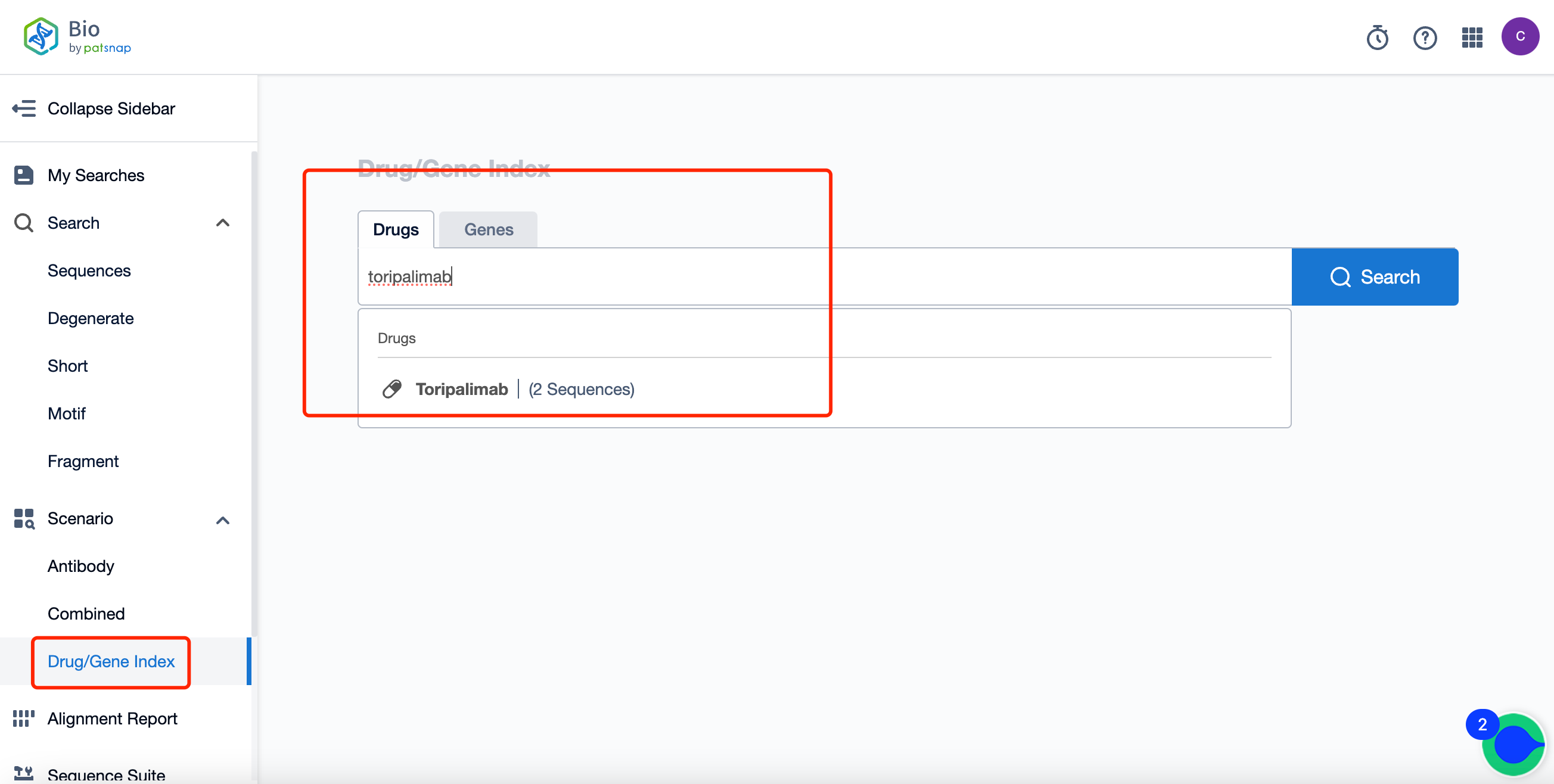
How Can You Acquire Comprehensive Information on Toripalimab Sequence Patents for Free?
Firstly, establish a free account with Patsnap Bio Sequence Database. Proceed to the homepage's "standard search," and enter the toripalimab sequence or directly input the drug name, toripalimab, in the "Drug/Gene index.” This single action will unravel extensive details of toripalimab's sequence, patent, literature, data from diversified sources, and a visually competitive landscape of patents.
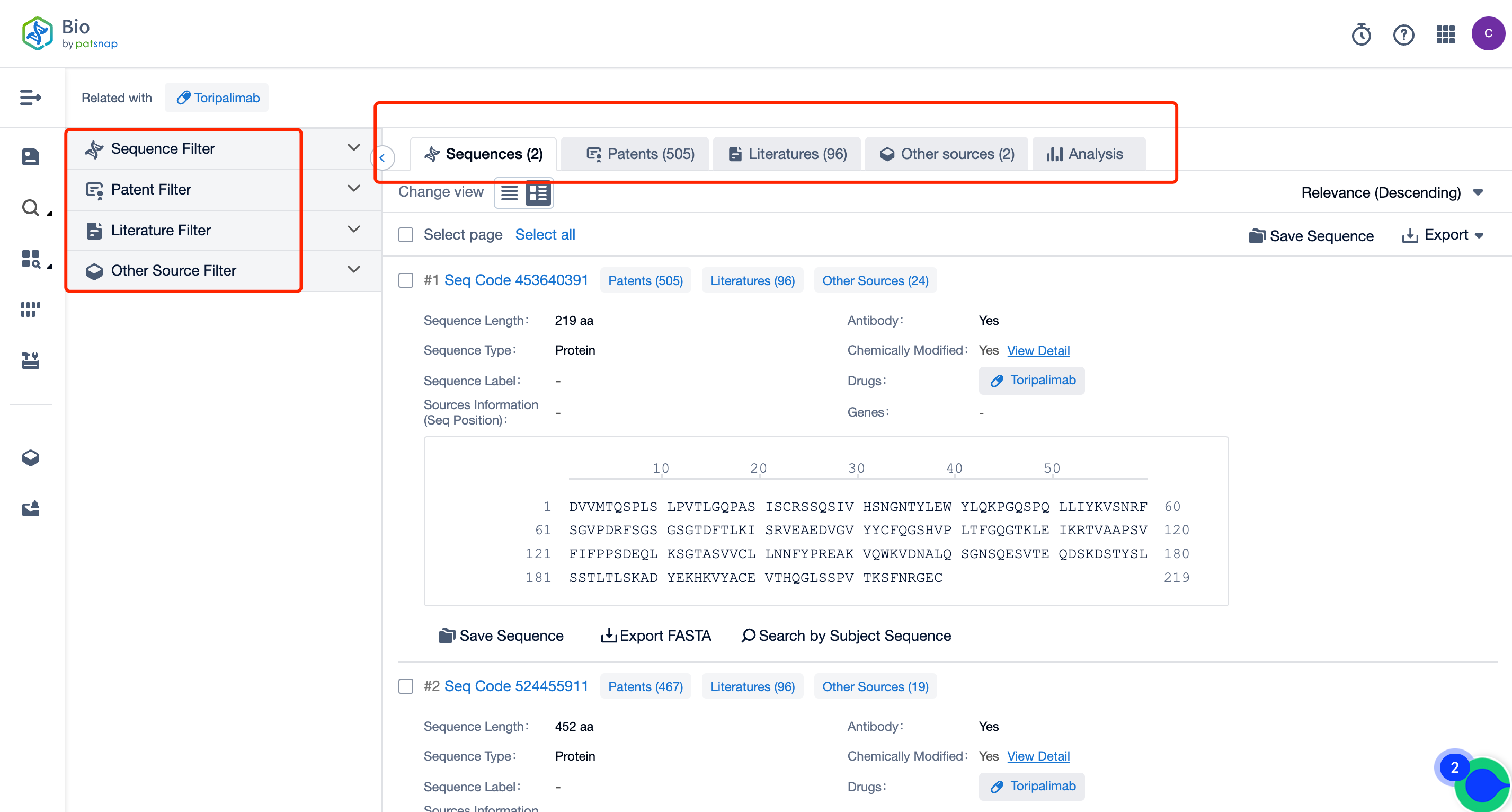
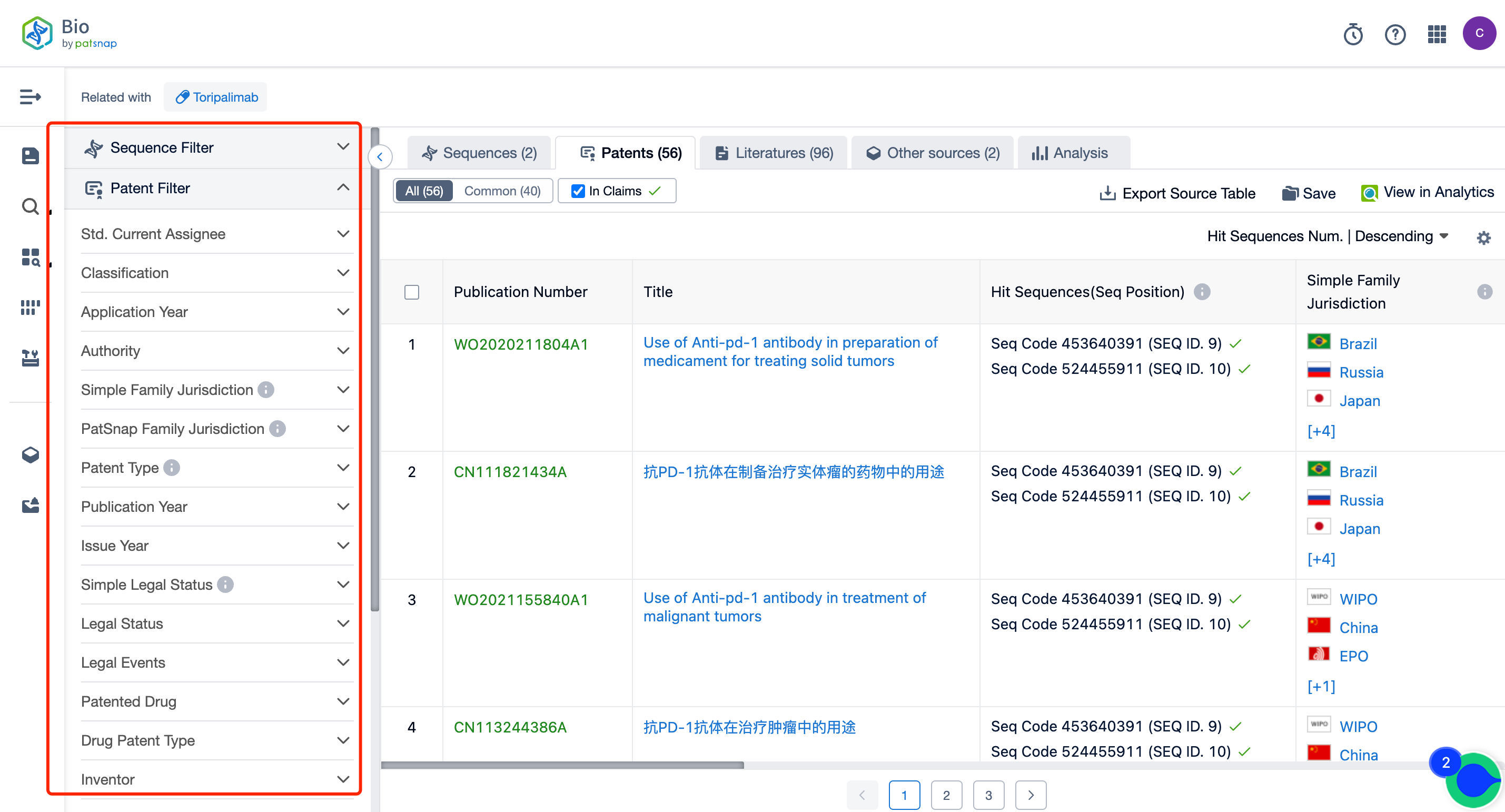
The left panel's search result details page is equipped with an exhaustive range of filters, enabling you to pinpoint specific data accurately, thereby boosting your search experience and overall efficiency. Clicking on each data point will unfold a rich and detailed data set and a host of advantageous and practical tools to support your research process.
It is important to note that Patsnap Bio is the most extensive sequence search platform for the Patsnap database. It incorporates AI with human-curated data for comprehensive handling of protein and nucleotide sequence data plucked from global patents, biological periodicals, and public repositories. Essential biological sequences are manually annotated, illuminating structural modifications to provide the most accurate sequence data and boost sequence retrieval efficiency.
Free registration is available for the Bio biological sequence database: https://bio.patsnap.com. Act now to expedite your sequence search tasks.