Ciltacabtagene autoleucel Unveiled: A Detailed Overview of its Revolutionary R&D Breakthroughs
Ciltacabtagene autoleucel's R&D Progress
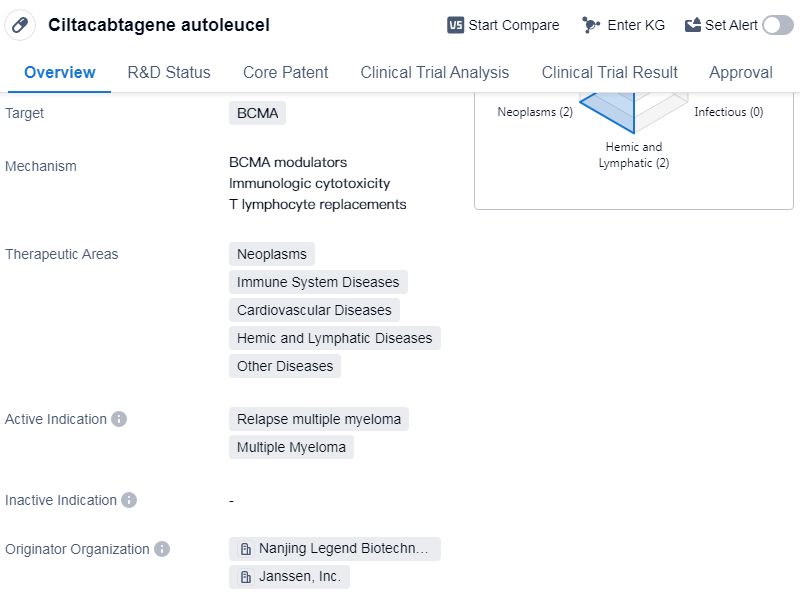
Ciltacabtagene autoleucel is a CAR-T therapy that targets B-cell maturation antigen (BCMA). It is primarily used in the treatment of neoplasms, immune system diseases, cardiovascular diseases, hemic and lymphatic diseases, and other diseases. The drug is specifically indicated for relapse multiple myeloma and multiple myeloma.
Ciltacabtagene autoleucel was developed by Nanjing Legend Biotechnology Co., Ltd. and Janssen, Inc. It has reached the highest phase of development, which is approved globally. In China, it is currently in the New Drug Application/Biologics License Application (NDA/BLA) phase.
The drug received its first approval in the United States in February 2022. It is regulated under various programs, including priority review, conditional marketing approval, breakthrough therapy, orphan drug, and special review project. These regulatory designations highlight the potential significance and urgency of the drug in addressing unmet medical needs.
CAR-T therapies, like ciltacabtagene autoleucel, are a promising approach in the field of biomedicine. They involve modifying a patient's own immune cells to recognize and attack cancer cells expressing specific antigens, such as BCMA in this case. This targeted therapy has shown remarkable efficacy in treating certain types of cancer, including multiple myeloma.
The approval of ciltacabtagene autoleucel represents a significant milestone in the treatment of relapse multiple myeloma and multiple myeloma. It provides a new therapeutic option for patients who have exhausted other treatment options or have not responded well to conventional therapies.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Ciltacabtagene autoleucel: BCMA modulators, Immunologic cytotoxicity and T lymphocyte replacements
BCMA modulators are substances or drugs that target and modulate the activity of B-cell maturation antigen (BCMA). BCMA is a protein that is primarily expressed on the surface of plasma cells, which are a type of white blood cell involved in the immune response. BCMA modulators can either enhance or inhibit the activity of BCMA, depending on their mechanism of action.
Immunologic cytotoxicity refers to the ability of the immune system to kill or destroy target cells through various mechanisms. This process involves the recognition of specific antigens on the surface of target cells by immune cells, such as T cells or natural killer (NK) cells. Once the target cells are recognized, the immune cells release cytotoxic molecules or engage in direct cell-to-cell contact to induce cell death.
T lymphocyte replacements refer to therapies or treatments aimed at replacing or replenishing T lymphocytes, also known as T cells. T cells are a type of white blood cell that plays a crucial role in the immune response, particularly in recognizing and eliminating infected or abnormal cells. T lymphocyte replacements can involve techniques such as adoptive cell transfer, where T cells are collected from a donor or engineered in the laboratory and then infused into a patient to enhance their immune response.
Drug Target R&D Trends for Ciltacabtagene autoleucel
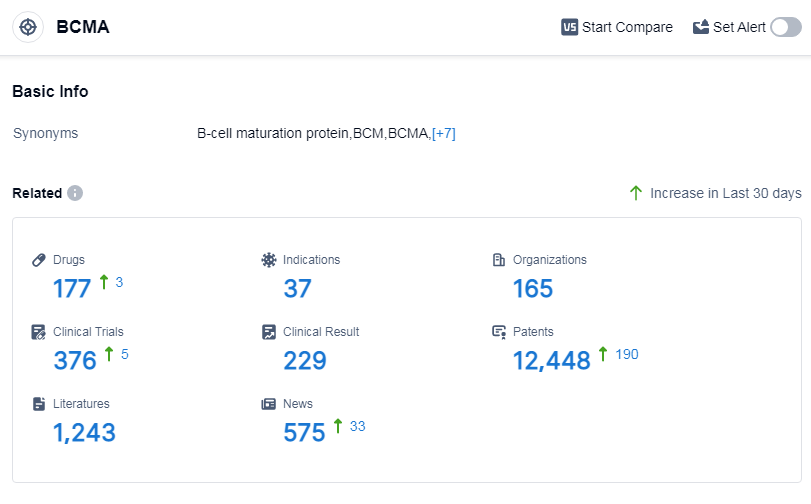
According to Patsnap Synapse, as of 15 Sep 2023, there are a total of 177 BCMA drugs worldwide, from 165 organizations, covering 37 indications, and conducting 376 clinical trials.
The analysis of the target BCMA in the pharmaceutical industry reveals several key insights. Johnson & Johnson, Bristol Myers Squibb Co., and Genscript Biotech Corp. are the companies with the highest number of drugs in various development phases. Multiple myeloma is the most common indication, with ongoing research and development in the Preclinical stage. CAR-T is the most prominent drug type, indicating a focus on innovative therapies and intense competition. The United States and China are leading in the development of target BCMA drugs, with China showing significant progress in the preclinical stage. Overall, the target BCMA presents a competitive landscape with potential for future development and advancements in the treatment of various indications.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Ciltacabtagene autoleucel is a CAR-T therapy that targets B-cell maturation antigen (BCMA). The drugwas developed by Nanjing Legend Biotechnology Co., Ltd. and Janssen, Inc. It has reached the highest phase of development, which is approved globally. In China, it is currently in the NDA/BLA phase.