NAYA Biosciences publishes new insights on CD38-targeting Flex-NK™ dual-specific antibody in American Society of Hematology journal
NAYA Biosciences Inc. has made a public statement revealing the release of recent findings about its innovative CD38-directed Flex-NK™ Bispecific Antibody, known as NY-338, formerly CYT-338. This promising therapeutic agent is being researched for its potential application in combating Multiple Myeloma. The company disclosed that the research information was included in an abstract format within the supplemental material of Blood journal, associated with the 2023 gathering of the American Society of Hematology. The important abstract falls under the category dedicated to discussing forward-looking treatment trials in relation to Multiple Myeloma.
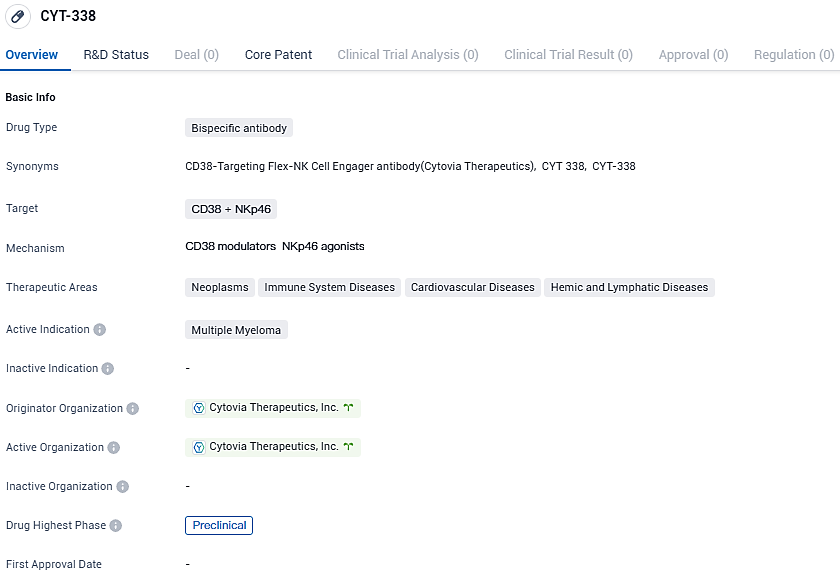
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"NAYA is in the process of building a distinctive collection of oncology therapeutics that are in the clinical phase. This collection includes two innovative FLEX-NK™ bispecific antibodies that were obtained from Cytovia Therapeutics," stated NAYA's CEO Dr. Daniel Teper. "The recent findings related to our CD38-targeted antibody are especially promising as they strive to overcome the drawbacks of existing treatments and propose novel therapeutic avenues for patients suffering from multiple myeloma."
Dr. Ola Landgren, MD, PhD, who co-authored the study and serves as Professor of Medicine, Myeloma Division Chief, and Experimental Therapeutics Program Leader at the Sylvester Comprehensive Cancer Center at the University of Miami, elaborated, "The collaborative activation of NK cells through NKp46 significantly boosts the immune response triggered by FLEX-NK™ bispecific antibodies. This enhancement not only reduces NK cell self-destruction but also sustains their numbers and increases their efficacy, which includes the ability to counter many dysfunctions that NK cells face."
The research findings for NY-338 reveal a distinction in its mechanism and potential superior rank when compared to the drug daratumumab. This distinction supports launching clinical trials by the year 2024. More precisely, the data underscores that NY-338's innovative activation of NKp46, which decreases NK cell self-destruction and preserves NK cell quantity, in addition to increasing efficacy including rectifying NK cell dysfunction, positions NY-338 as a premium contender as an anti-CD38 therapy for MM when contrasted with daratumumab.
These insights advocate the progression of CYT-338 as a standalone treatment and as part of a regimen that includes other immunotherapeutic agents under development or already sanctioned for MM. Plans are underway for a pioneering Phase 1 trial in the United States, aimed at individuals dealing with MM that has either relapsed or become refractory to treatment.
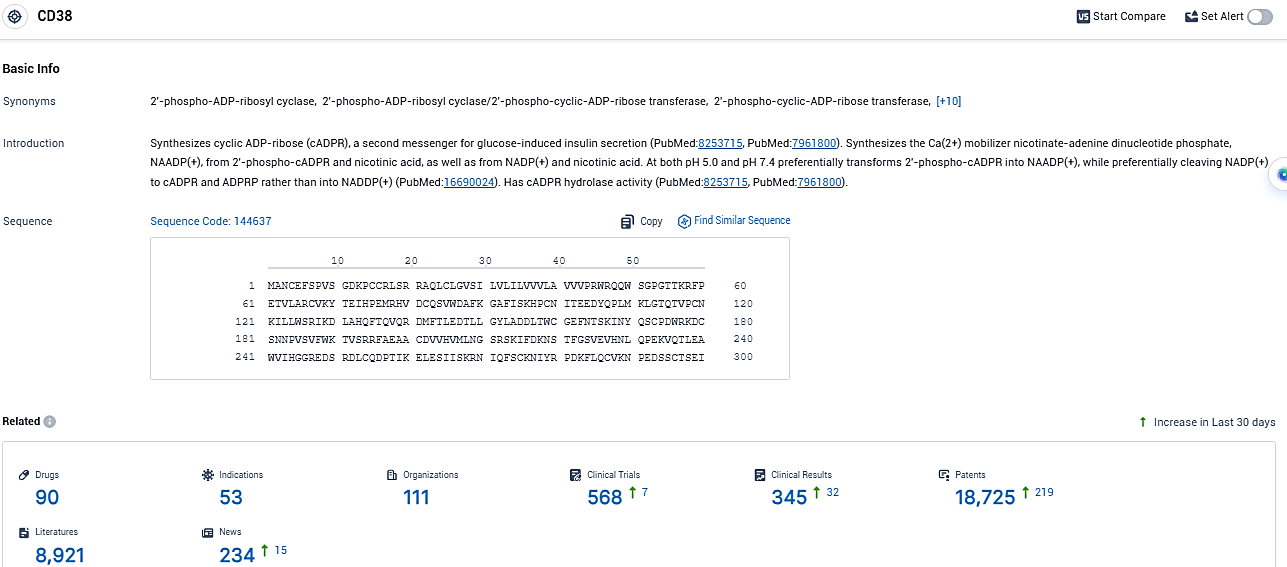
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 17, 2023, there are 90 investigational drugs for the CD38 target, including 53 indications, 111 R&D institutions involved, with related clinical trials reaching 568, and as many as 18725 patents.
CYT-338 targets CD38 proteins and is primarily intended for the treatment of multiple myeloma. The drug is currently in the preclinical phase, and further studies are needed to determine its safety and efficacy in humans.