IASO Bio and Innovent released new data on Equecabtagene Autoleucel (FUCASO®) for multiple myeloma at ASH 2023
IASO biotechnology together with Innovent Biologics, Inc., disclosed new analytical outcomes from their FUMANBA-1 trial examining the efficacy of Equecabtagene Autoleucel in addressing multiple myeloma. They shared these findings during a spoken report at the 65th yearly convergence of the American Society of Hematology.
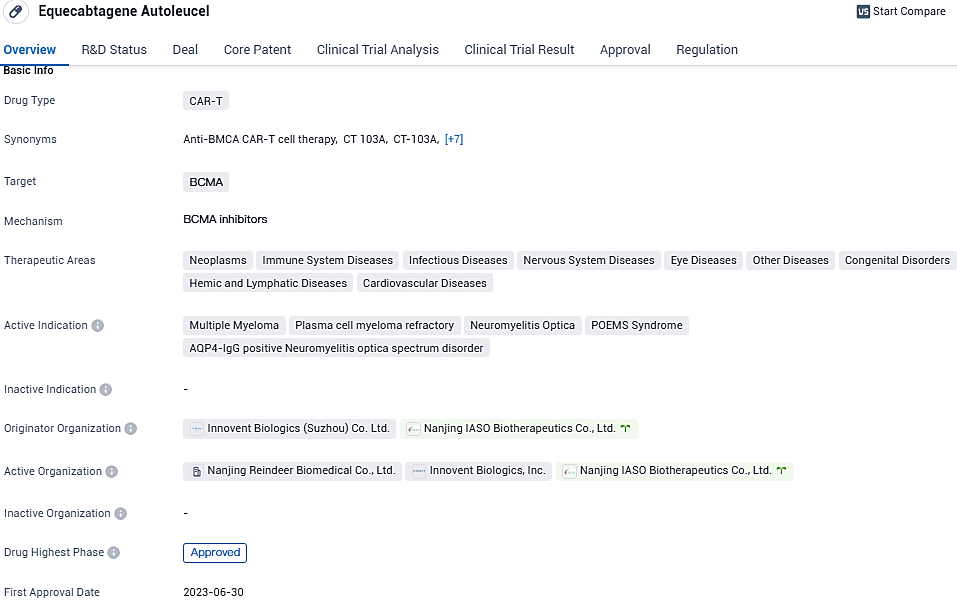
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The session underscored the effectiveness and unique aspects of a fully human B-cell maturation antigen (BCMA)-specific CAR-T therapy in achieving sustained remission from minimal residual disease in treated multiple myeloma cases.
This report is rooted in a secondary examination of the results from the clinical trial known as FUMANBA-1. The FUMANBA-1 trial is characterized as a Phase Ib/II, single-cohort, multi-site research initiative designed to evaluate the potency and security of the investigational medicinal product called Equecabtagene Autoleucel. This human-derived CAR-T cell treatment has been tested on subjects with advanced stages of multiple myeloma who have undergone at least three other therapy forms.
As the data stood on the last day of 2022, following approximately an 18-month median observation period, profound and enduring therapeutic responses were recorded across 103 assessable participants. Within this group, the general rate of positive treatment responses reached 96.1%, with strict complete responses or complete responses in 77.7% of cases. For patients not previously treated with CAR-T, the overall response rate climbed to 98.9%, with a stringent complete response/complete response tally of 82.4% and a one-year survival without disease progression standing at 85.5%.
Observations from the study indicate that treatment with eque-cel resulted in MRD clearance regardless of factors like genetic makeup of the cancer, presence of disease outside the bone marrow, the extent of previous treatment, or the patient's clinical condition. This implies eque-cel's robust capacity to eliminate myeloma cells, proving to be independent of these variables.
"MRD status is acknowledged as a critical marker influencing the survival rates of patients with relapsed and refractory multiple myeloma (RRMM). With the ability to persist effectively in the body, Equecabtagene Autoleucel paves the way for durable, deep remission in RRMM patients, even after several therapies have failed. This development holds significant promise for improved treatment outcomes," communicated Professor Lu-gui Qiu from the Institute of Hematology and Blood Diseases Hospital at Huazhong University of Science & Technology, a lead researcher in the study.
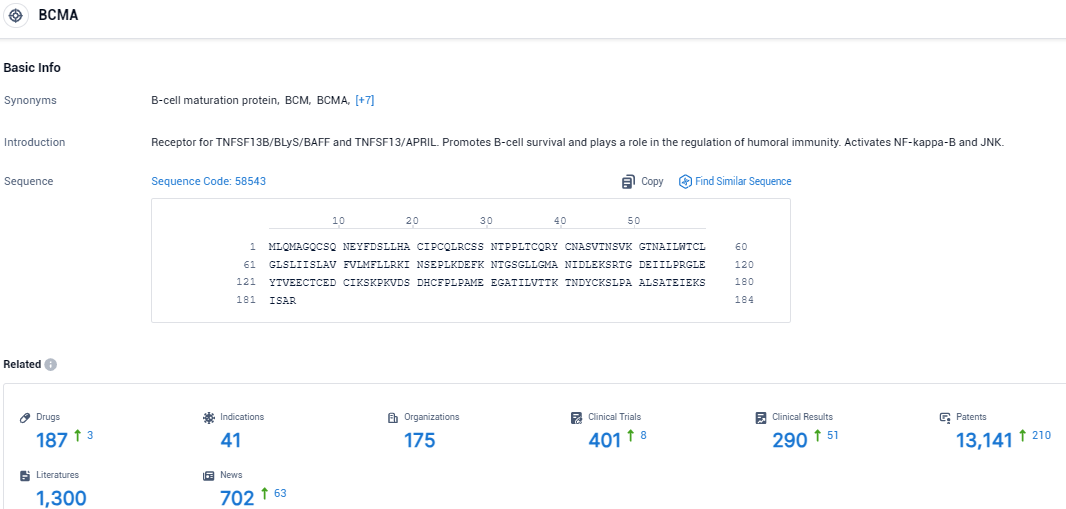
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 17, 2023, there are 187 investigational drugs for the BCMA target, including 41 indications, 175 R&D institutions involved, with related clinical trials reaching 401, and as many as 13141 patents.
Equecabtagene Autoleucel is a CAR-T therapy that targets BCMA and has shown promising results in the treatment of multiple diseases. Its approval in China and globally, along with the various regulatory designations it has received, underscore its potential as an innovative and impactful treatment option in the field of biomedicine.