Neurotech Pharmaceuticals' NT-501 (revakinagene taroretcel) BLA for Macular Telangiectasia Type 2 Gets Priority Review
Neurotech Pharmaceuticals, Inc., an innovator in sustained drug delivery for chronic retinal diseases, announces that the U.S. Food and Drug Administration, has determined that the Biologic License Application for NT-501, an investigational encapsulated cell therapy for the treatment of MacTel, is sufficiently complete to permit a substantive review. The application has a prescription drug user fee act goal date of December 17, 2024.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
MacTel is a progressive, neurodegenerative disease of the retina that results in the deterioration of central vision, significantly impacting patients’ quality of life. NT-501 is an ocular implant designed to deliver sustained therapeutic doses of ciliary neurotrophic factor (CNTF) directly to the retina to slow the progression of the disease.
MacTel, or idiopathic juxtafoveal macular telangiectasia, is a rare neurodegenerative disease with characteristic localized retinal degeneration with secondary alterations of the retinal vasculature.1 MacTel typically affects both eyes and causes deterioration in central vision impacting patients’ quality of life.
The ECT platform is a cell-based delivery system that was designed to provide long-term, sustained delivery of therapeutic proteins for the treatment of chronic retinal diseases. This versatile platform consists of a small, semi-permeable capsule containing proprietary allogeneic RPE cells genetically engineered to produce specific therapeutic proteins for targeted disease treatment. The capsule is surgically inserted into the patient’s vitreous and sutured to the sclera during an outpatient procedure.
Once in place, the capsule’s semi-permeable membrane allows essential nutrients to enter, while also permitting the therapeutic proteins to exit into the vitreous, providing targeted and continuous treatment. Simultaneously, the membrane protects the encapsulated RPE cells from the host’s immune system, ensuring their long-term survival and functionality.
NT-501 leverages our ECT platform to deliver CNTF for the treatment of chronic retinal diseases, such as MacTel. CNTF is a potent neuroprotective protein that promotes the survival and maintenance of photoreceptors. This targeted therapy provides sustained delivery of CNTF, aiming to slow retinal degeneration and potentially improve long-term visual outcomes for patients.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
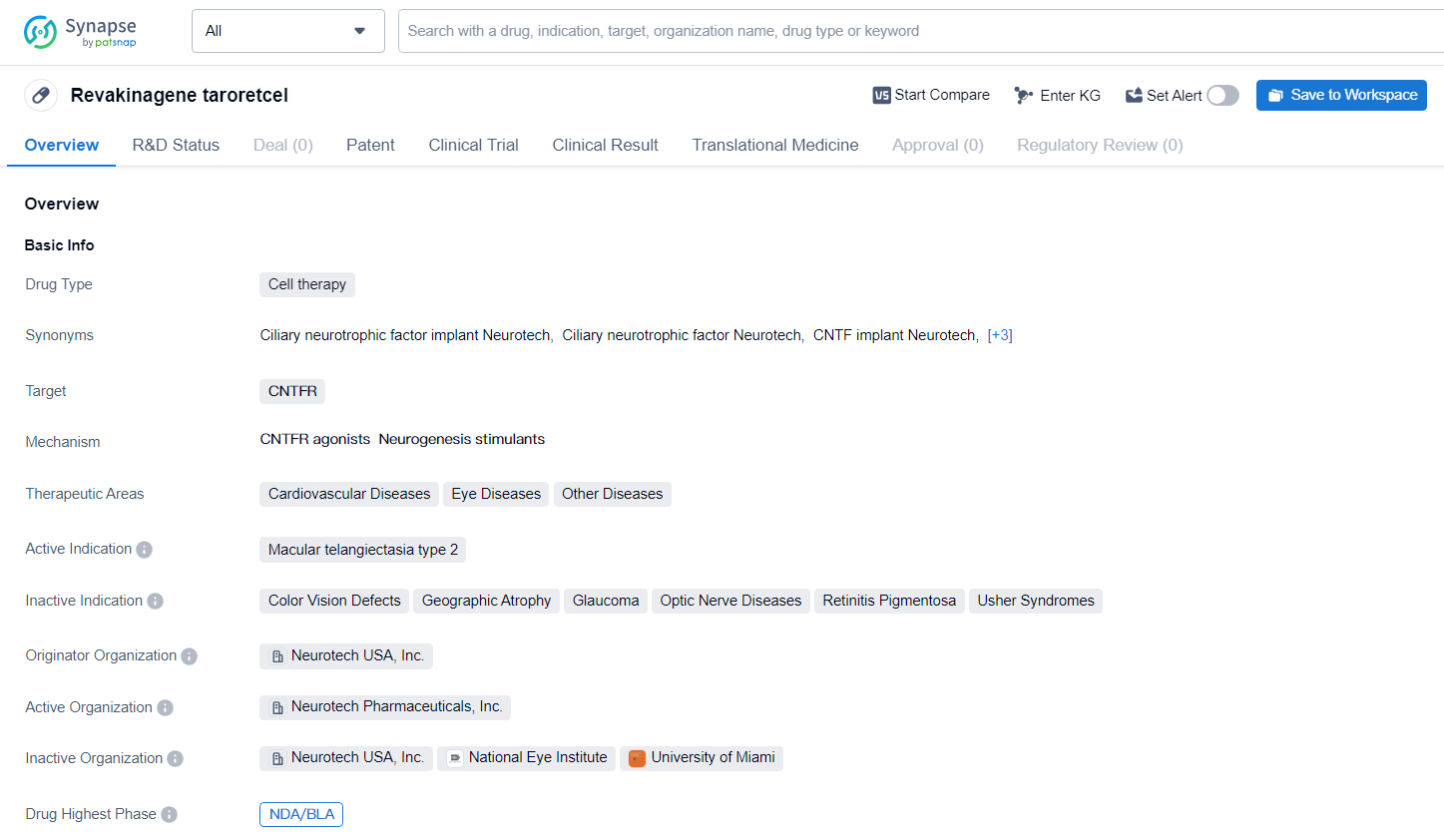
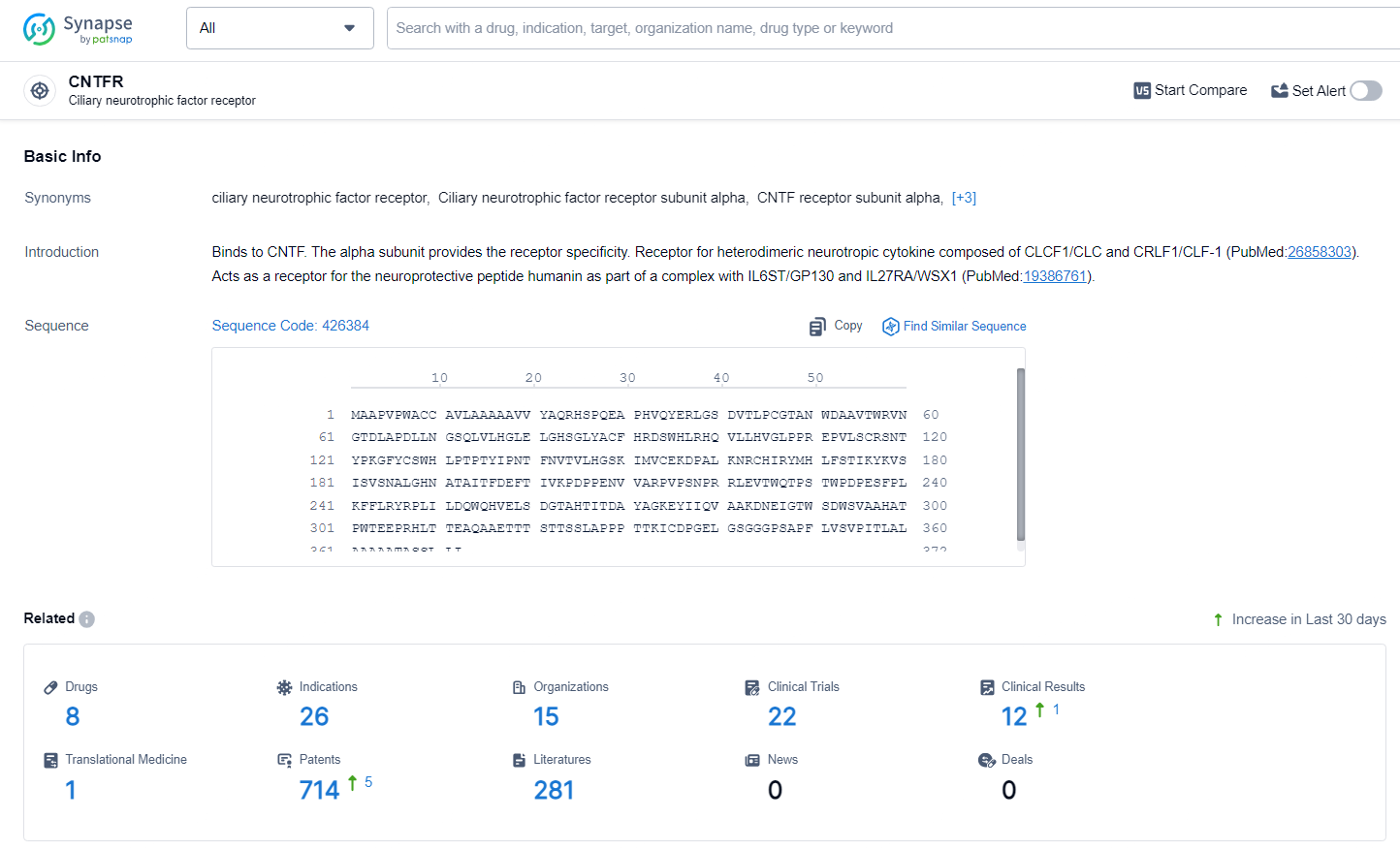
According to the data provided by the Synapse Database, As of June 24, 2024, there are 8 investigational drugs for the CNTFR target, including 26 indications, 15 R&D institutions involved, with related clinical trials reaching 22, and as many as 714 patents.
NT-501 targets CNTFR and is being developed for the treatment of various therapeutic areas including cardiovascular diseases, eye diseases, and other diseases. The active indication for NT-501 is macular telangiectasia type 2. NT-501 represents a significant advancement in the field of biomedicine, particularly in the areas of cardiovascular and eye diseases. The drug's potential to address macular telangiectasia type 2, a condition affecting the macula of the eye, holds promise for patients suffering from this specific indication.