InnoCare Submission Accepted for Tafasitamab-Lenalidomide BLA for Adult Relapsed/Refractory DLBCL in China
InnoCare Pharma, a leading biopharmaceutical company focusing on the treatment of cancer and autoimmune diseases, announced that China National Medical Products Administration has accepted and granted priority review to a biologics license application for tafasitamab in combination with lenalidomide for adult patients with relapsed or refractory diffuse large B-cell lymphoma who are not eligible for autologous stem cell transplant.
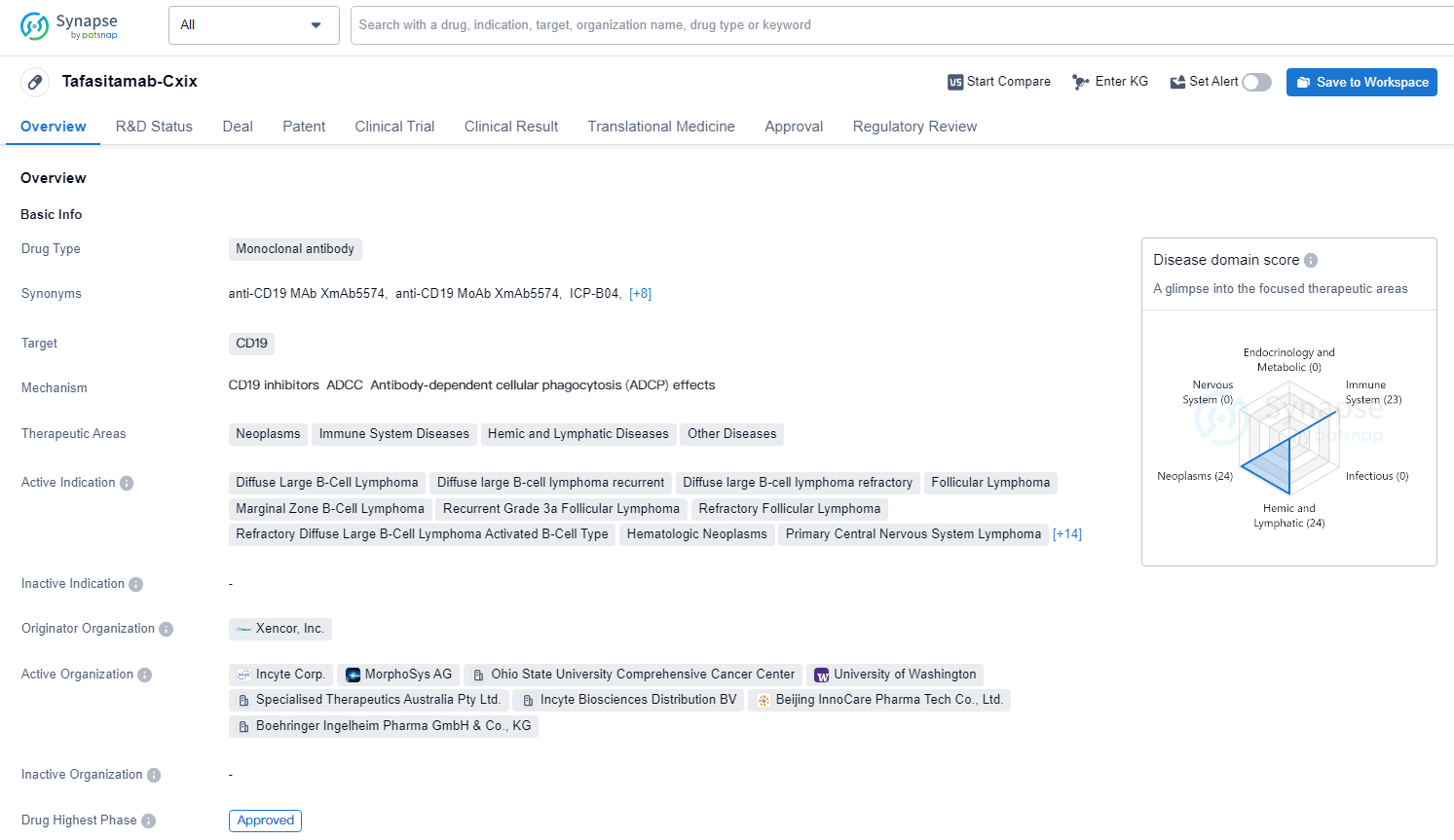
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Dr. Jasmine Cui, Co-founder, Chairwoman and CEO of InnoCare, said, “The BLA acceptance marks an important milestone for InnoCare. DLBCL is the most common type of non-Hodgkin lymphoma globally, and there is a significant unmet need for DLBCL patients in China. We believe that tafasitamab regimen will bring a novel treatment therapy to DLBCL patients in China.”
Tafasitamab, a humanized Fc-modified cytolytic CD19-targeting immunotherapy, in combination with lenalidomide has already been approved for the treatment of eligible DLBCL patients in Hong Kong. Furthermore, under the early access program in the Bo’ao Lecheng International Medical Tourism Pilot Zone and the Guangdong-Hong Kong-Macao Greater Bay Area, prescriptions of tafasitamab in combination with lenalidomide were issued at the Ruijin Hainan Hospital and Guangdong Clifford Hospital for eligible DLBCL patients.
Tafasitamab is approved under accelerated approval by the U.S. Food and Drug Administration, and conditional approved by the European Medicines Agency, in combination with lenalidomide for the treatment of relapsed or refractory DLBCL adult patients who are not eligible for ASCT.
Tafasitamab is a humanized Fc-modified cytolytic CD19 targeting monoclonal antibody. In 2010, MorphoSys licensed exclusive worldwide rights to develop and commercialize tafasitamab from Xencor, Inc. Tafasitamab incorporates an XmAb engineered Fc domain, which mediates B-cell lysis through apoptosis and immune effector mechanism including Antibody-Dependent Cell-Mediated Cytotoxicity and Antibody-Dependent Cellular Phagocytosis.
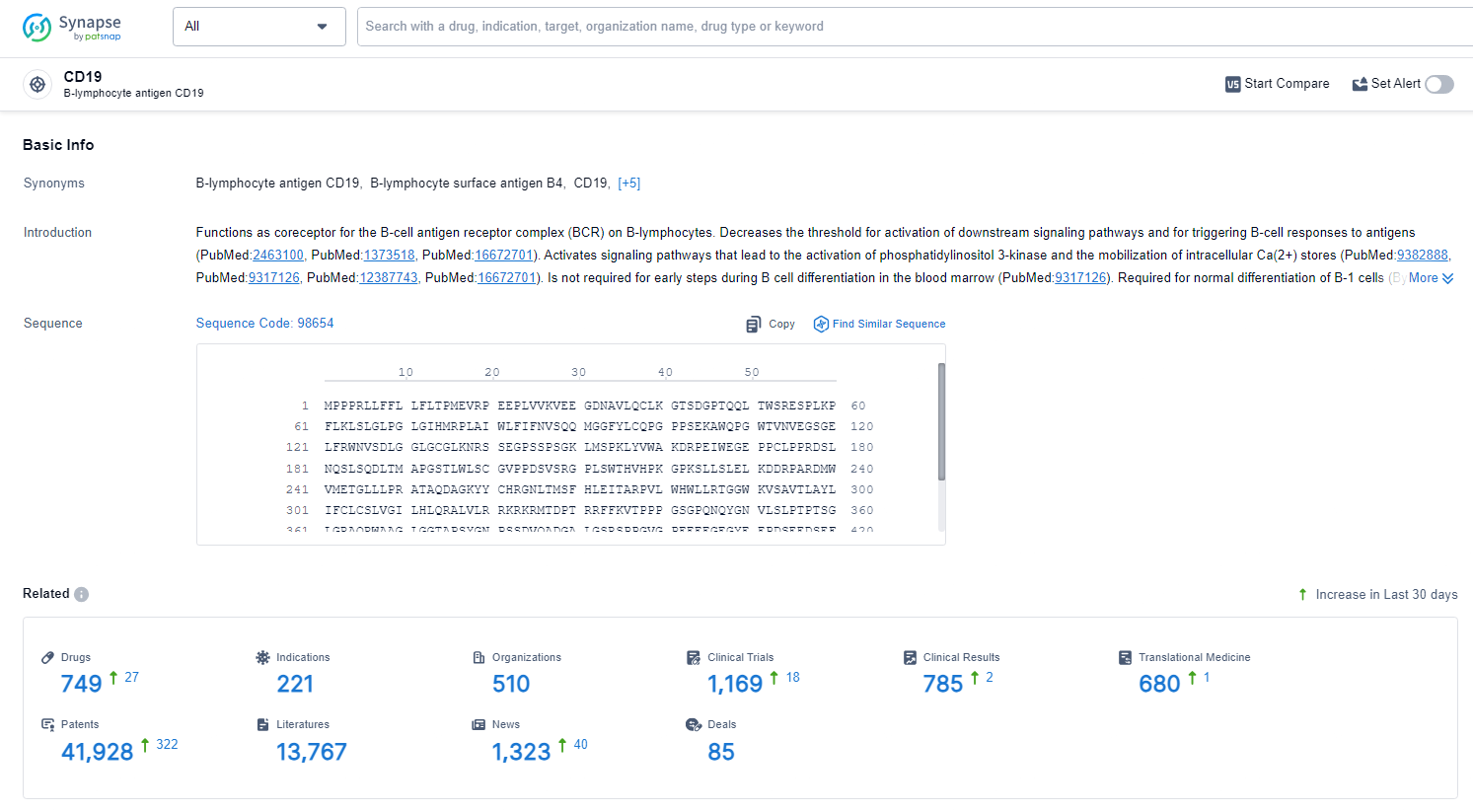
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 24, 2024, there are 749 investigational drugs for the CD19 target, including 220 indications, 511 R&D institutions involved, with related clinical trials reaching 1167, and as many as 41924 patents.
Tafasitamab-Cxix targets CD19 and is indicated for the treatment of various neoplastic, immune system, and hemic and lymphatic diseases. The drug has been approved for use in the United States since July 2020 and has received conditional marketing approval, accelerated approval, fast track designation, breakthrough therapy designation, and orphan drug status.