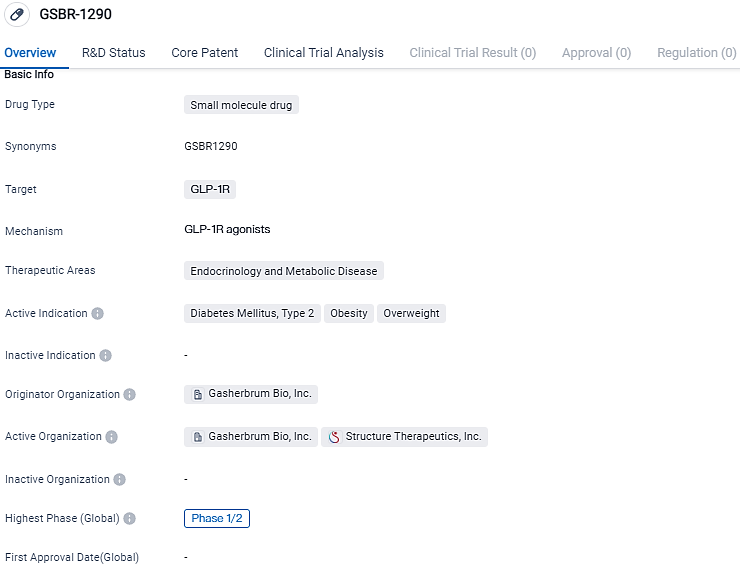
Structure Therapeutics reports outcomes from the Phase 1b clinical study of GSBR-1290
Structure Therapeutics Inc., a worldwide biopharmaceutical firm in the clinical stage that creates new small molecule oral treatments for cardiopulmonary and metabolic conditions, has revealed encouraging results from its Phase 1b incremental dose study of its exclusive oral GLP-1 receptor agonist, GSBR-1290, in overweight or obese individuals. The 28-day trial showed that GSBR-1290 led to substantial weight reduction, supported once-a-day dosage, and presented a positive safety and tolerability profile.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"The encouraging data from the first phase of the GSBR-1290 project gives us confidence in its potential as an oral GLP-1 receptor agonist that requires once-daily dose," stated Ray Stevens, Ph.D., the founder and chief executive of Structure. "We are enthusiastic to reveal outcomes of GSBR-1290's 12-week stretch in the subsequent Phase 2a test and we persist in our efforts to initiate Phase 2b trials specific to both type 2 diabetes and obesity, as scheduled, in 2024."
During the Phase 2a testing, an oversight at one of the clinical sites resulted in absent data pertaining to the obesity group where the final visit weight was not recorded for 24 out of 40 participants. However, all safety measures and lab tests were conducted during all visits, including the 12-week mark as per the prescribed protocol. Structure will recruit additional subjects for this cohort of the Phase 2a trial to offset the lack of 12-week weight data.
The newcomers will be subject to the identical study procedures, with no amendments to the titration pattern or intended dosage. As a result, Structure anticipates revealing comprehensive data from the obesity sector in early 2024. Despite the company being blind to the data from Phase 2a obesity group, none of the 40 participants ceased the study due to adverse events by the end of the 12-week term.
Structure stays focused on delivering comprehensive data from the type 2 diabetes cohort of the ongoing Phase 2a testing in the closing period of the fourth quarter of 2023 along with the findings from GSBR-1290’s ethno-bridging study in Japan.
Structure is preparing to kickstart two additional Phase 2b tests involving GSBR-1290 in 2024. It's expected that the type 2 diabetes project will involve nearly 500 patients throughout the US, Europe, and Japan. Simultaneously, the obesity project is projected to enlist around 275 participants from across the United States and Europe.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
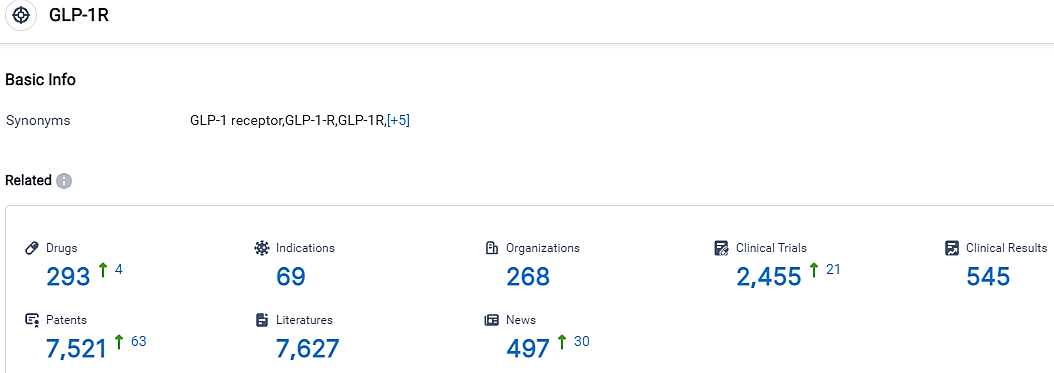
According to the data provided by the Synapse Database, As of October 9, 2023, there are 293 investigational drugs for the GLP-1R target, including 69 indications, 268 R&D institutions involved, with related clinical trials reaching 2455,and as many as 7521 patents.
GSBR-1290 targets the GLP-1R and is indicated for the treatment of diabetes mellitus, type 2, obesity, and overweight. With its focus on endocrinology and metabolic disease, GSBR-1290 has the potential to address significant health challenges and improve patient outcomes. However, further research and clinical trials are needed to determine its safety and efficacy in larger patient populations.