Allogene Therapeutics Presents Promising ALLO-316 Phase 1 Data for Advanced Renal Cell Carcinoma at SITC and IKCS
Allogene Therapeutics, Inc. (Nasdaq: ALLO), a biotechnology company in the clinical development phase, is at the forefront of creating allogeneic CAR T (AlloCAR T™) therapies for cancer and autoimmune disorders. The company will share new findings from the Phase 1 TRAVERSE trial through an oral presentation at the 2024 International Kidney Cancer Symposium (IKCS) and during a poster session at the Annual Meeting of The Society for Immunotherapy of Cancer (SITC). This trial investigates ALLO-316, Allogene's initial AlloCAR T product candidate, targeting solid tumors. The Phase 1 TRAVERSE trial, still recruiting, includes patients with advanced or metastatic renal cell carcinoma (RCC) who have not responded to treatments involving immune checkpoint inhibitors and VEGF-targeted therapies. These presentations showcase significant evidence of CAR T mechanism activity and anti-tumor effectiveness in 26 RCC patients with CD70-positive tumors who were assessed for efficacy results.
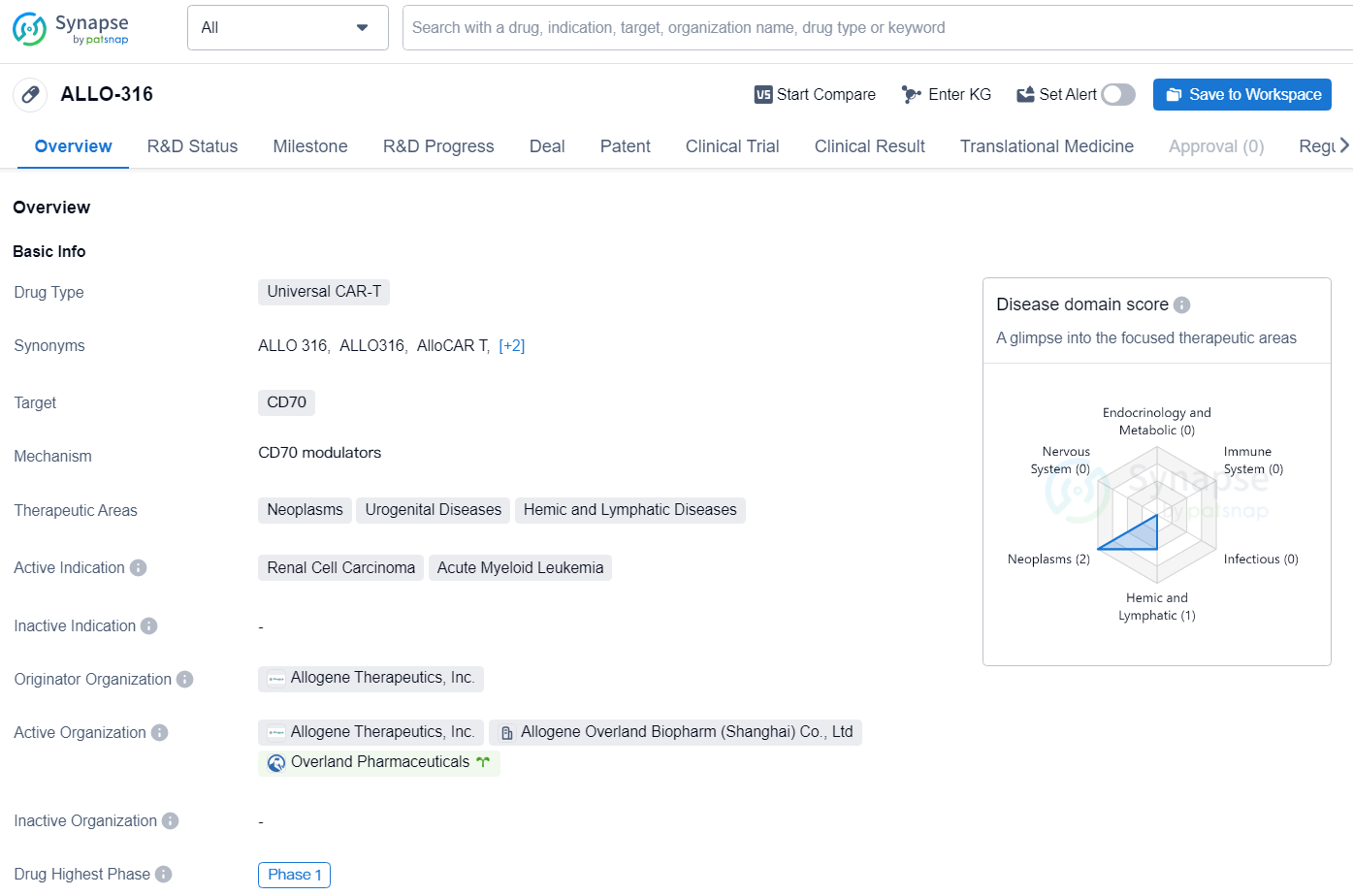
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
ALLO-316, the top "off-the-shelf" CAR T therapy under exploration for solid tumors, is exhibiting extraordinary effectiveness in the TRAVERSE study. According to Zachary Roberts, M.D., Ph.D., EVP, Research and Development and Chief Medical Officer at Allogene, data from the Phase 1 trial reveals significant anti-tumor impacts in individuals with metastatic disease resistant to various treatment classes, even after standard lymphodepletion. This could signify a substantial progression in the field. "The exceptional cell expansion and endurance driven by CD70 CAR-intrinsic Dagger® technology, along with compelling evidence of tumor penetration by CAR T cells, underscores the unique characteristics of ALLO-316. We anticipate that these findings from the Phase 1 trial will establish the foundation for a novel generation of allogeneic cell therapies."
By the data cutoff on October 14, 2024, 39 participants had joined the ongoing Phase 1 trial, with 26 patients confirmed to have CD70 positive RCC and assessable for efficacy results. The median duration from enrollment to therapy commencement was five days. Presentations include data from dose escalation cohorts and the progressing Phase 1b expansion cohort. The Phase 1b expansion cohort is investigating the safety and efficacy of ALLO-316 at DL2 (80M CAR T cells) following the conventional FC500 (fludarabine (30 mg/m2/day) and cyclophosphamide (500 mg/m2/d) for three days) lymphodepletion regimen. Eventually, this cohort is projected to involve around 20 patients. Further data from the Phase 1b expansion cohort is anticipated to be released in mid-2025.
After a single ALLO-316 infusion in patients subjected to extensive prior treatments, the trial recorded a maximum Overall Response Rate (ORR) of 50% and a Confirmed Response Rate of 33% among those with a CD70 Tumor Proportion Score (TPS) of ≥50% who received DL2. Patients with a TPS of ≥50% represent the majority of those with advanced or metastatic RCC. Among those with a TPS of ≥50, 76% (16/21) experienced a reduction in tumor burden. Of the six patients with high TPS who received the Phase 1b expansion regimen, two (33%) showed ongoing durable responses at ≥4 months.
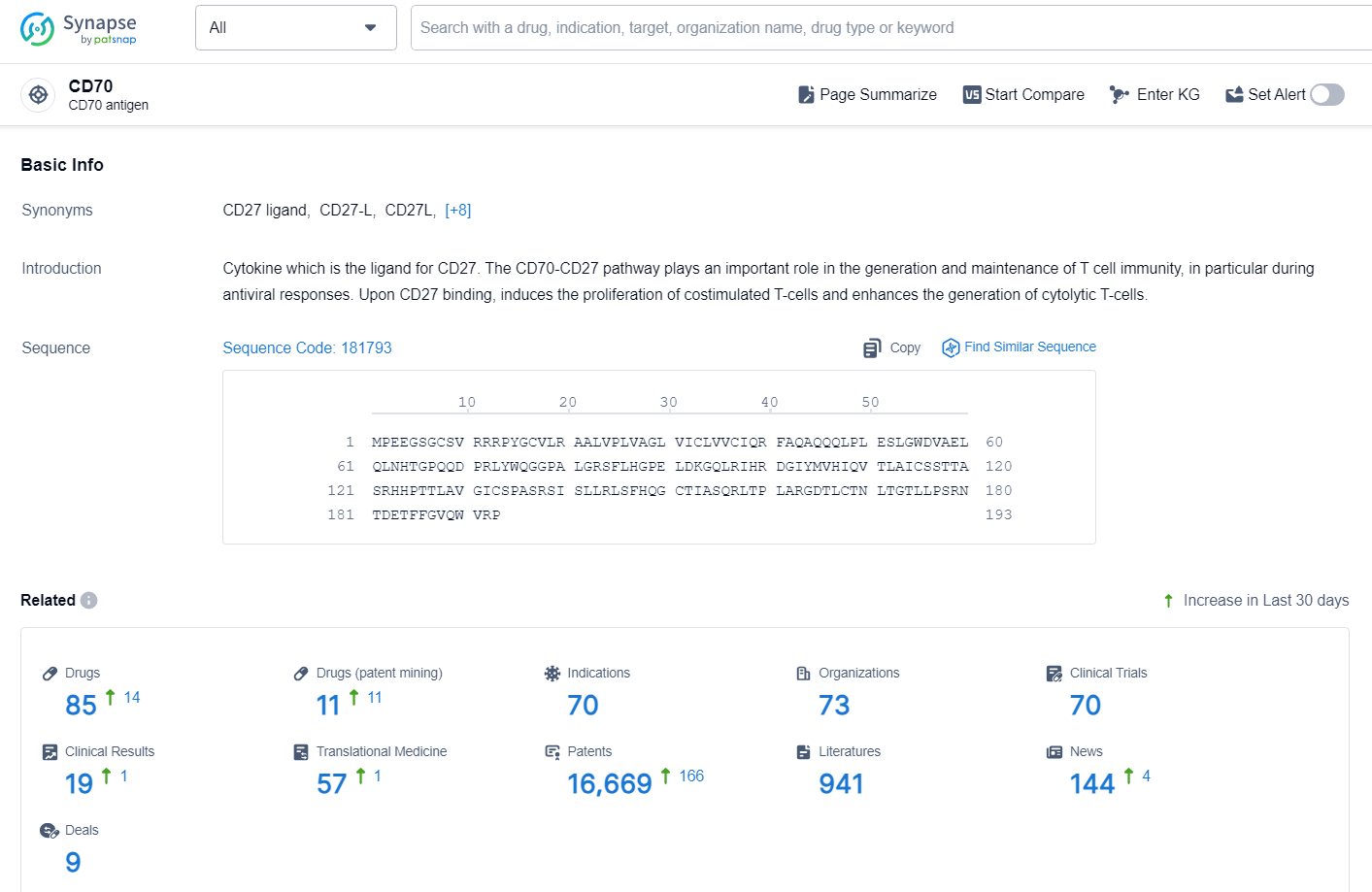
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of November 13, 2024, there are 85 investigational drugs for the CD70 target, including 70 indications, 73 R&D institutions involved, with related clinical trials reaching 70, and as many as 16669 patents.
ALLO-316 is a Universal CAR-T drug developed by Allogene Therapeutics, Inc. It targets CD70 and is intended for the treatment of neoplasms, urogenital diseases, and hemic and lymphatic diseases. The active indications for ALLO-316 include renal cell carcinoma and acute myeloid leukemia.