Nkarta Launches Myasthenia Gravis Study and Opens Ntrust-2 Enrollment
Nkarta, Inc. (Nasdaq: NKTX), a biopharmaceutical firm focused on developing engineered natural killer (NK) cell therapies, has announced the commencement of patient enrollment for Ntrust-2 and has received IND approval for an investigator-initiated trial (IST) aimed at assessing NKX019, Nkarta's allogeneic, CD19-targeted chimeric antigen receptor (CAR) NK-cell therapy, in individuals diagnosed with myasthenia gravis (MG). The Ntrust-2 trial, which is multi-centered, will examine NKX019 in three simultaneous cohorts, involving patients with systemic sclerosis (SSc, scleroderma), idiopathic inflammatory myopathy (IIM, myositis), and ANCA-associated vasculitis (AAV). The IST will be conducted under the direction of researchers from the University of California, Irvine, and the University of Kansas Medical Center.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
NKX019 represents a next-generation, allogeneic, off-the-shelf chimeric antigen receptor (CAR) NK-cell therapeutic option designed to target and eliminate CD19-expressing cells in disorders driven by B-cells. This innovative approach harnesses the inherent advantages of NK cell-based treatments, such as the ability for rapid destruction of B-cells without requiring expansion of the cells, a diminished likelihood of adverse effects typically associated with swift cell proliferation, avoidance of fludarabine in lymphodepletion to decrease side effects, and flexibility in administration, allowing for repeat doses as necessary.
Paul J. Hastings, CEO of Nkarta, noted, “Expanding into these four additional autoimmune conditions, alongside our ongoing clinical trials for lupus nephritis and systemic lupus erythematosus, reflects the significant potential of our investigational NK cell therapy, NKX019, to offer a safe and readily available treatment alternative for those suffering from autoimmune diseases.”
Ntrust-2 is a multi-center, open-label, dose-escalation clinical study following academic research that demonstrated durable, drug-free remissions in patients experiencing autoimmune diseases after CD19-targeted therapies. This study will include patients diagnosed with systemic sclerosis (SSc), idiopathic inflammatory myopathies (IIM), or ANCA-associated vasculitis (AAV) in parallel groups. According to the study protocol, participants will receive NKX019 on Days 0, 3, and 7 after undergoing lymphodepletion with cyclophosphamide, which has a well-documented safety record in the context of autoimmune disorders. The primary objectives are to evaluate the safety of NKX019 and its capacity to facilitate long-term remissions by resetting the immune response through the removal of harmful B-cells.
The Phase 1 Investigator-Sponsored Trial (IST), which adopts a dual-center, single-arm, open-label design, will be overseen by Ali A. Habib, M.D., Clinical Professor of Neurology at the University of California, Irvine (UCI), along with other co-investigators.
Dr. Habib commented on the ongoing need for advancements in treatment for myasthenia gravis, stating, “Although there have been improvements in therapies that enhance health outcomes for myasthenia gravis patients, there is still a significant demand for better results and more efficient treatment delivery. Many existing therapies necessitate prolonged and potentially lifelong use. Cell therapy may offer a shift away from chronic treatment scenarios, ultimately transforming care approaches for those with myasthenia gravis.”
The IST will focus on enrolling individuals with myasthenia gravis and will study both safety and clinical efficacy. Additionally, translational research efforts such as examining autoantibody levels, cytokine profiles, and pharmacokinetics are planned. Patients will be administered NKX019 on Days 0, 3, and 7 following single-agent lymphodepletion using cyclophosphamide.
Myasthenia gravis (MG) is characterized as an autoimmune condition that disrupts communication between nerves and muscles, arising when B-cells in the immune system generate antibodies that obstruct or damage the neuromuscular junction. This condition can lead to muscle weakness and fatigue with symptoms that may fluctuate in intensity, and in severe cases, MG can compromise respiratory muscles. There remains no definitive cure for MG, and conventional treatment often necessitates ongoing use of immunosuppressive drugs.
Preliminary results from Ntrust-1 and Ntrust-2 are expected to be available in 2025. As previously reported, the first patient has been treated in Ntrust-1, which is a clinical trial evaluating NKX019 for lupus nephritis, in addition to an IST focused on systemic lupus nephritis at Columbia University Irving Medical Center. Both studies are currently open for participant enrollment.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
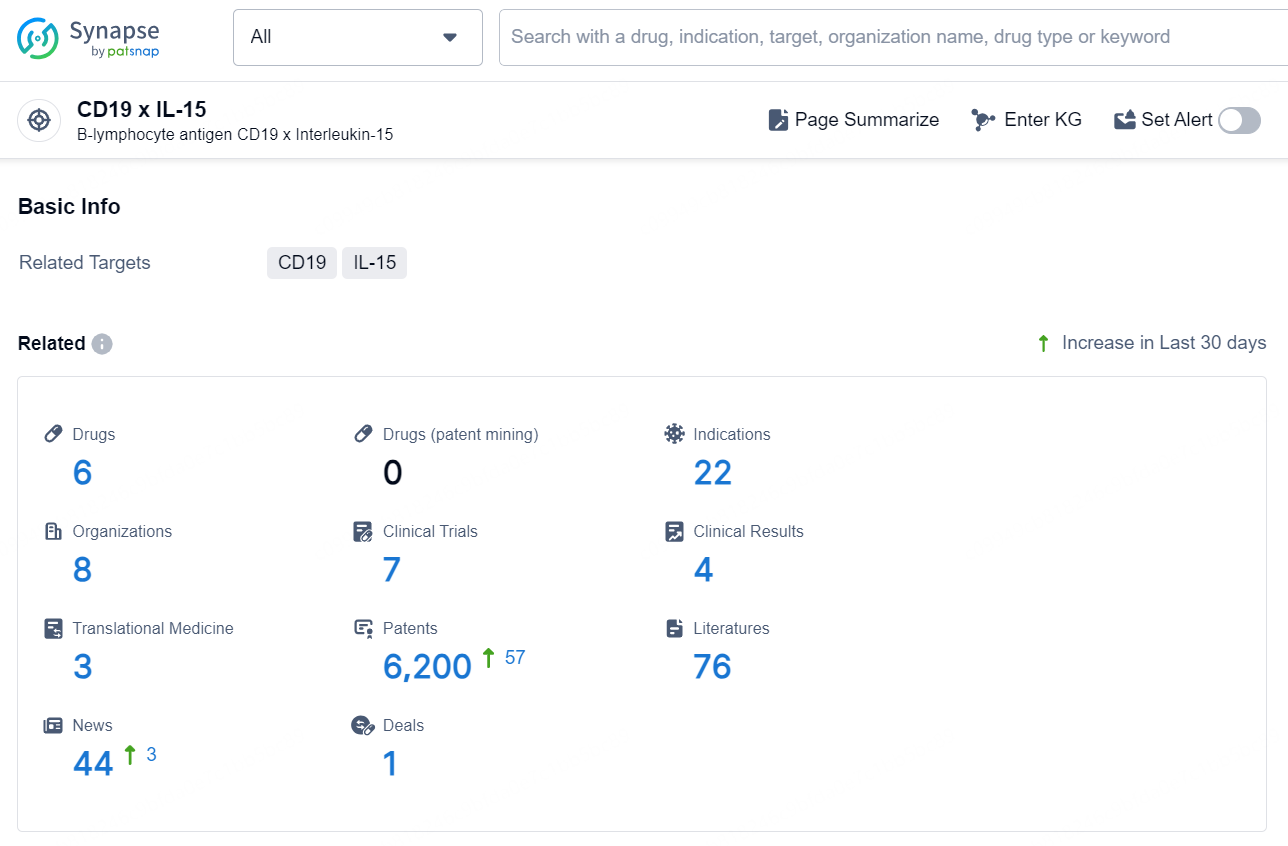
According to the data provided by the Synapse Chemical, As of December 10, 2024, there are 6 investigational drugs for the CD19 x IL-15 target, including 22 indications, 8 R&D institutions involved, with related clinical trials reaching 7, and as many as 6200 patents.
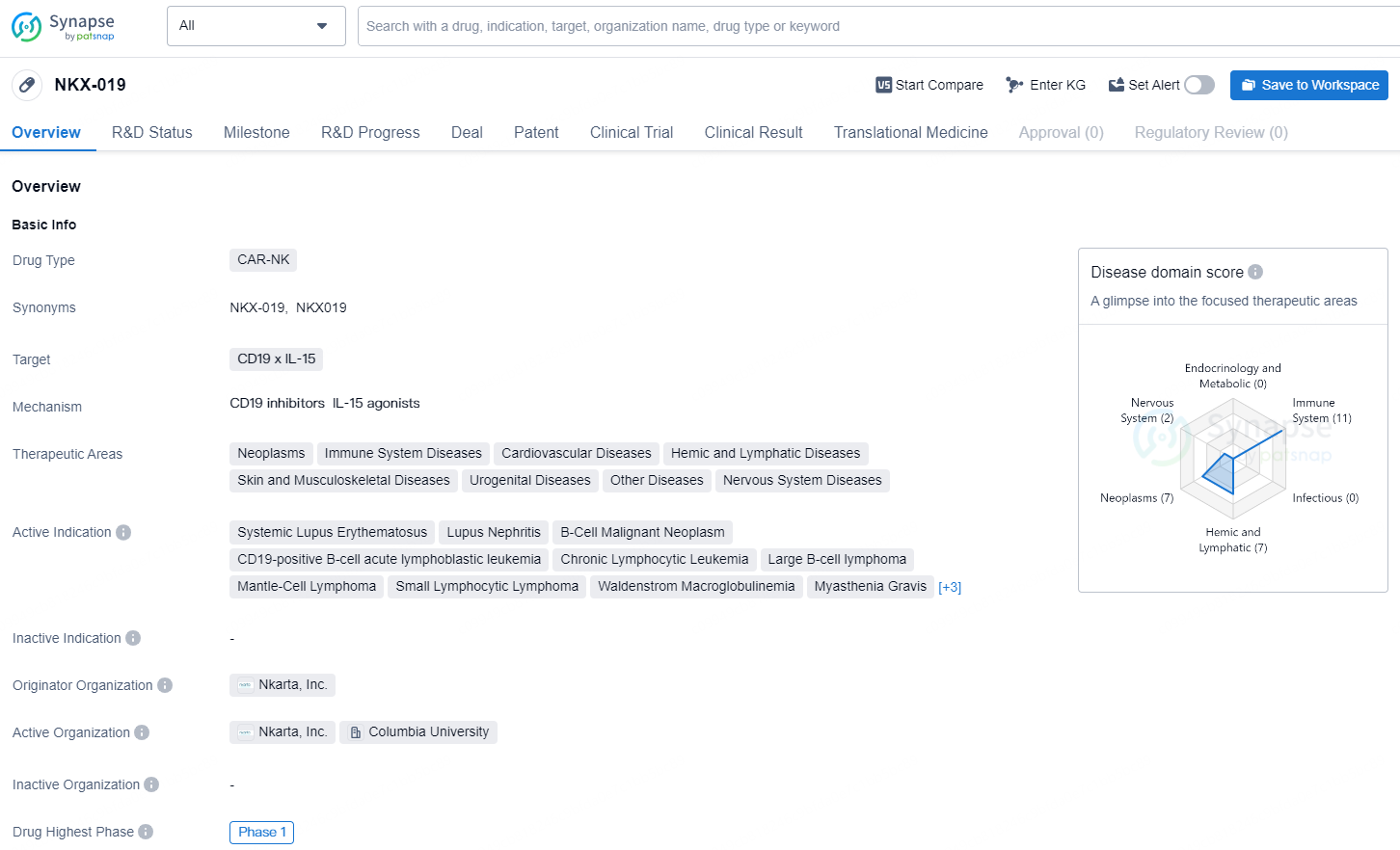
NKX-019 is a CAR-NK drug developed by Nkarta, Inc. The drug targets CD19 x IL-15 and is primarily focused on treating a wide range of diseases within therapeutic areas including neoplasms, immune system diseases, cardiovascular diseases, hemic and lymphatic diseases, skin and musculoskeletal diseases, urogenital diseases, other diseases, and nervous system diseases.