NMPA Approves Innovent's Picankibart for Moderate to Severe Plaque Psoriasis Treatment
Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a leading biopharmaceutical firm that focuses on the development, production, and commercialization of top-notch medications for oncology, autoimmune, cardiovascular and metabolic diseases, ophthalmology, and other significant medical conditions, has announced that the New Drug Application (NDA) for picankibart injection, a recombinant anti-interleukin 23p19 subunit (IL-23p19) antibody, has been accepted by the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) in China. This application pertains to the treatment of moderate to severe plaque psoriasis. Picankibart stands out as the first IL-23p19 antibody drug to demonstrate that more than 80% of participants achieved PASI 90 after 16 weeks of treatment during a registrational Phase 3 clinical trial. Furthermore, it offers the longest maintenance dosing interval (once every 12 weeks) among similar biologics. This drug is anticipated to deliver extensive benefits, such as significant skin lesion clearance, enhanced convenience of medication, and an improved quality of life for Chinese patients suffering from moderate to severe plaque psoriasis.
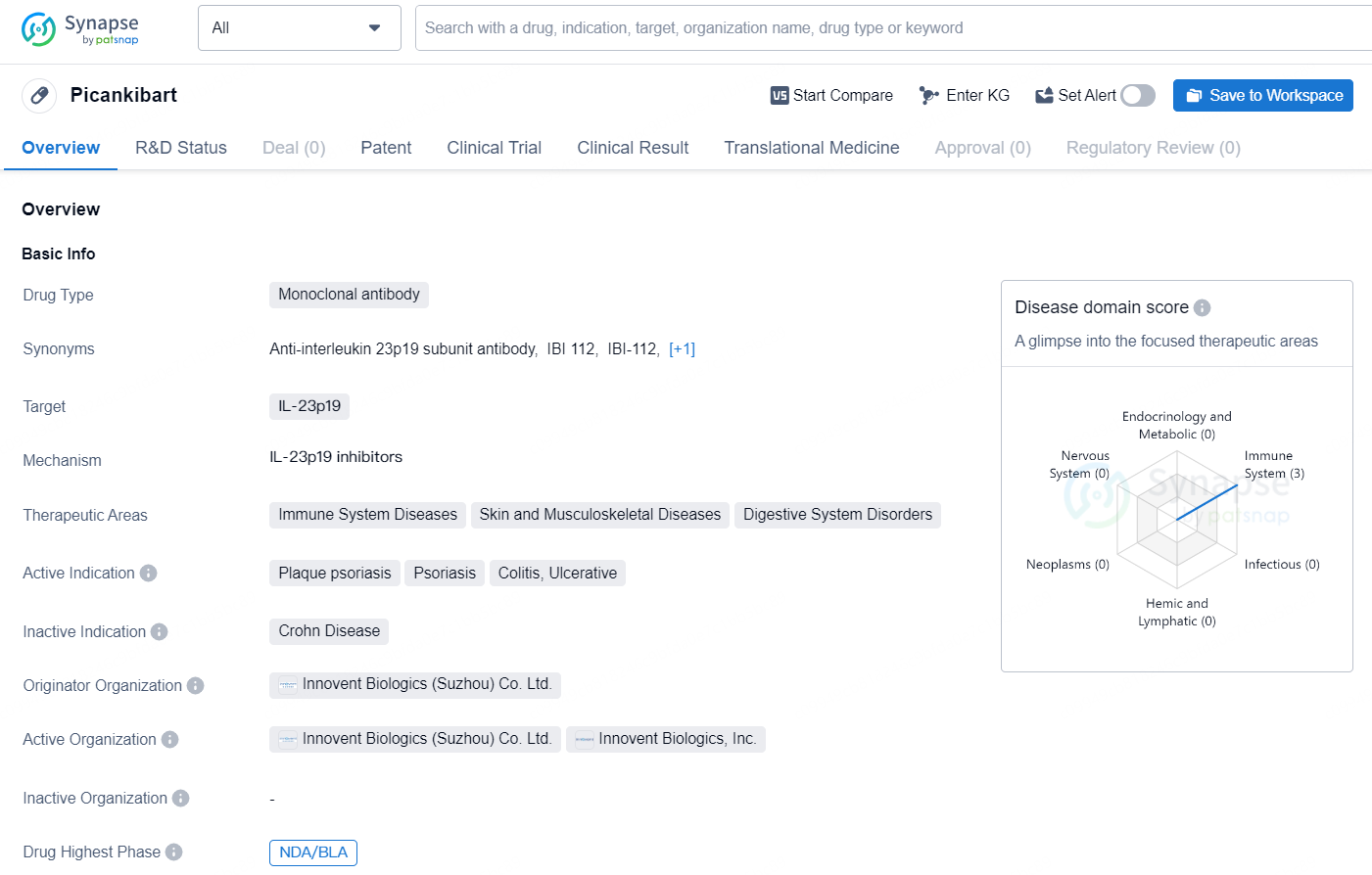
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
This NDA approval is grounded on positive outcomes from the Phase 3 registrational clinical trial CLEAR-1 (NCT05645627) conducted among Chinese subjects experiencing moderate to severe plaque psoriasis. The study reached its primary and significant secondary goals in May 2024, demonstrating greatly superior skin lesion clearance rates (PASI 90, PASI 75, PASI 100, sPGA 0/1, and sPGA 0) and enhancements in quality of life (DLQI 0/1) in the picankibart cohort compared to the placebo group. Picankibart exhibited a positive safety profile with no new safety concerns. Detailed findings from the CLEAR-1 study are set to be shared at upcoming academic symposiums and published in scholarly journals.
Professor Yulin Shi, the main researcher of the clinical trial from Shanghai Skin Disease Hospital, expressed, “Psoriasis is a persistent, non-curable condition that affects both the physical and mental wellness of patients, diminishing their quality of life. Opting for effective treatment approaches is crucial for managing the disease, lowering comorbidities, maintaining long-term therapeutic effectiveness, and improving overall life quality. Research suggests IL-23p19-targeted antibodies offer benefits in sustaining long-term efficacy and convenience in therapy, which Picankibart has distinctly shown in trials. Together with my colleagues in the study, I am pleased with its NDA submission in China, and we are optimistic for the approval of picankibart to aid Chinese psoriasis patients.”
Dr. Lei Qian, Vice President of Clinical Development at Innovent, commented, “We are privileged to have secured the regulatory acceptance of the NDA for picankibart, thanks to the dedication of participants, researchers, and regulatory bodies. This milestone signifies the first IL-23p19 antibody drug independently developed by a Chinese company to submit an NDA in China. Considering its effectiveness, safety, and long-interval maintenance dosing, picankibart showcases best-in-class potential. We will sustain active dialogue with regulatory authorities throughout the NDA review, striving to provide a secure and effective treatment option for Chinese patients with moderate to severe psoriasis. Additionally, we focus on product lifecycle management and will further explore the clinical value of picankibart through various clinical studies to address unmet needs for patients facing drug resistance or relapse. Innovent remains committed to enhancing its innovative portfolio in ophthalmology, autoimmune diseases, and cardiovascular and metabolic (CVM) conditions, with the goal of helping more individuals lead healthier lives.”
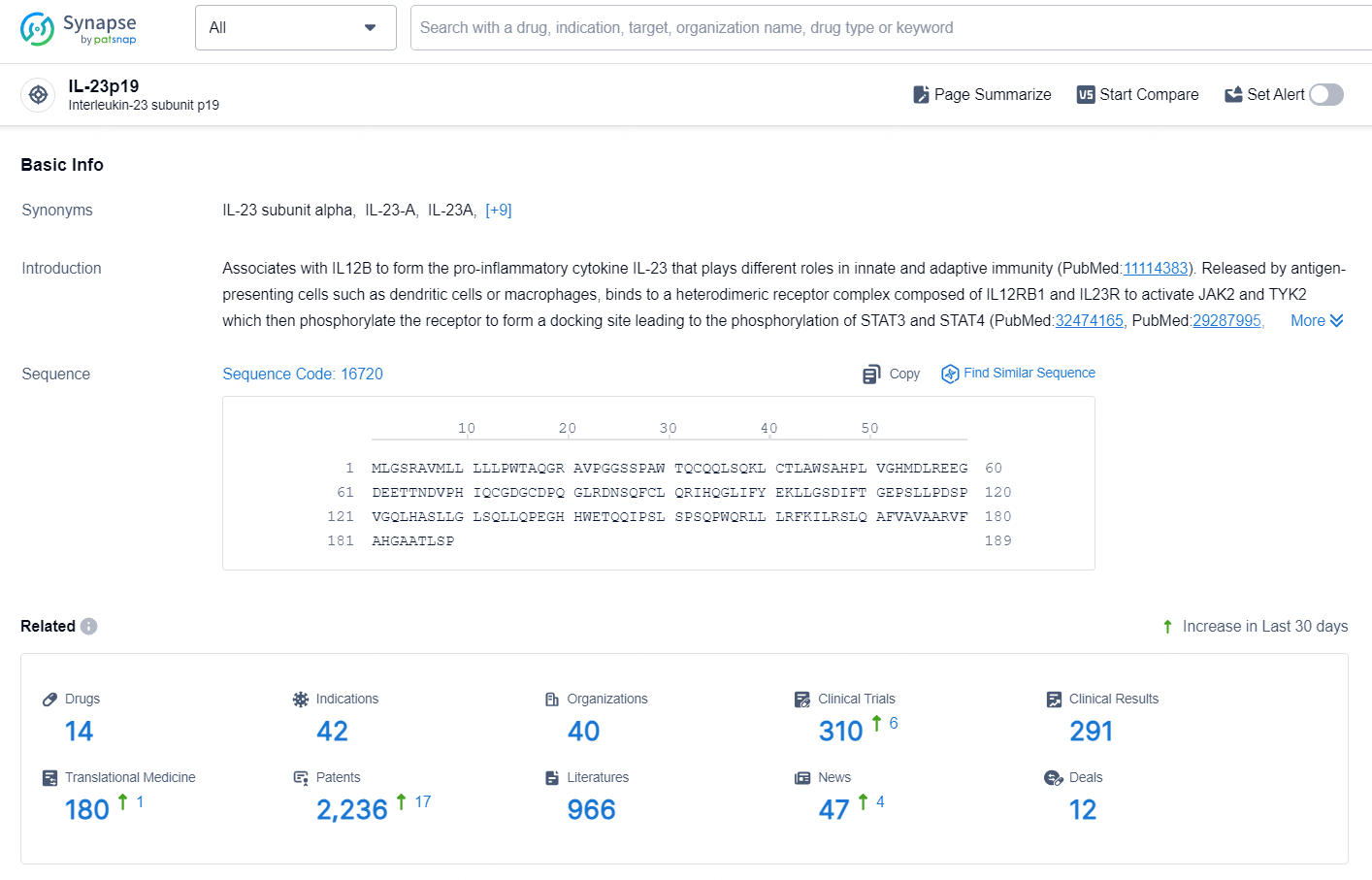
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 29, 2024, there are 14 investigational drug for the IL-23p19 target, including 42 indications, 40 R&D institutions involved, with related clinical trials reaching 310, and as many as 2236 patents.
The drug Picankibart is a monoclonal antibody that targets IL-23p19. It is indicated for the treatment of immune system diseases, skin and musculoskeletal diseases, and digestive system disorders. The active indications for Picankibart include plaque psoriasis, psoriasis, colitis, and ulcerative colitis. The drug is developed by Innovent Biologics (Suzhou) Co. Ltd., a biopharmaceutical company with a focus on the discovery, development, manufacturing, and commercialization of high-quality biologics for the treatment of major diseases. In terms of development, the drug has reached the highest phase of NDA/BLA (New Drug Application/Biologics License Application) both globally and in China.