Pfizer Recalls Global Batches of Sickle Cell Drug OXBRYTA®
Pfizer Inc. (NYSE: PFE) revealed its decision to voluntarily recall every batch of OXBRYTA® (voxelotor), used for treating sickle cell disease (SCD), across all regions where it has received approval. Moreover, Pfizer will be halting all ongoing clinical trials and expanded access initiatives for voxelotor on a global scale.
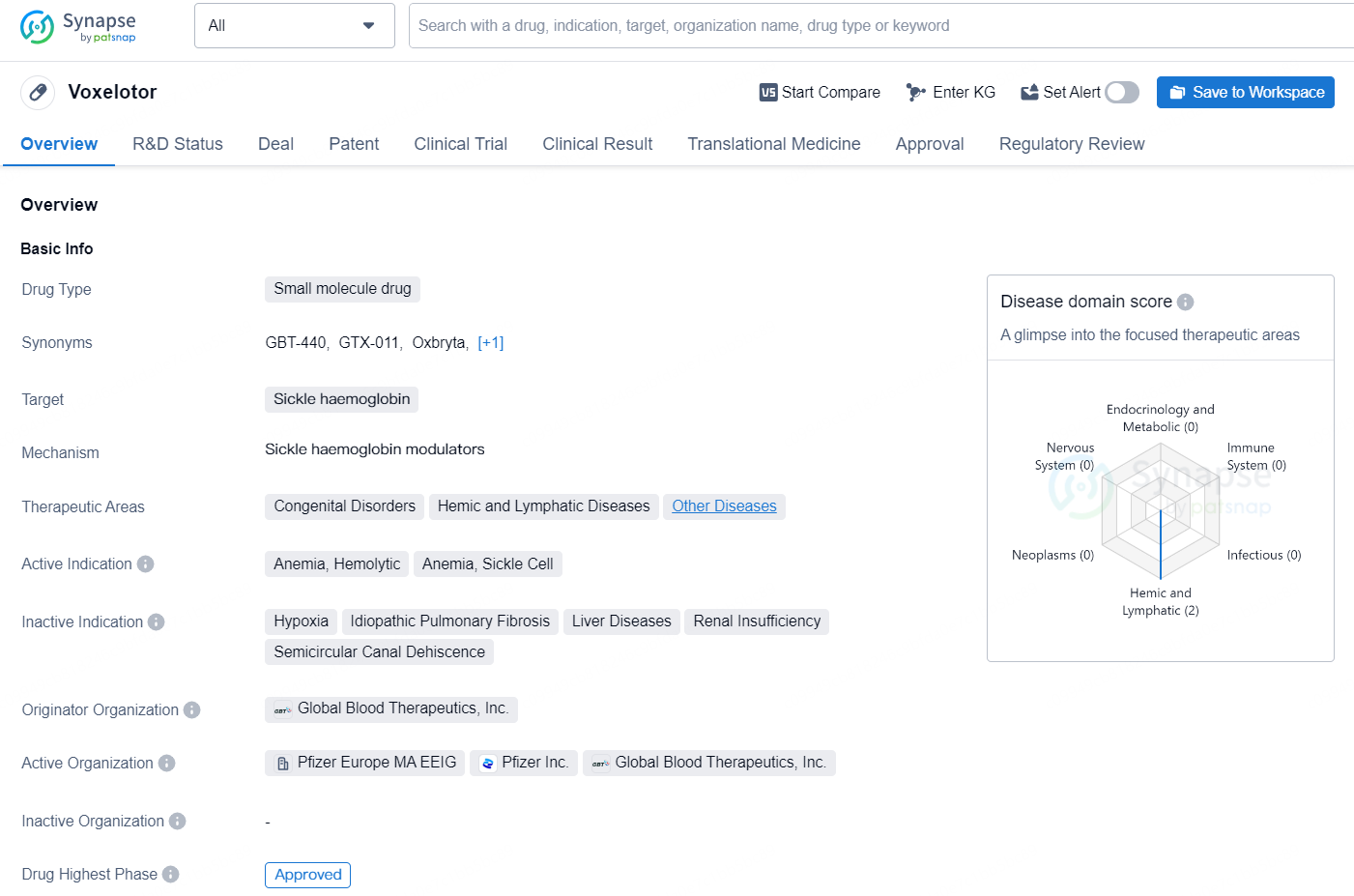
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Pfizer’s decision hinges on the entirety of clinical data currently suggesting that the overall benefit-risk profile of OXBRYTA no longer favors its use in the authorized sickle cell disease (SCD) patient group. The data indicate a discrepancy in vaso-occlusive crises and lethal events that necessitates further examination. Pfizer has alerted regulatory authorities about these results and has chosen to voluntarily pull OXBRYTA from the market, ceasing its distribution and clinical trials while reassessing the data and investigating the findings.
"The safety and well-being of patients are Pfizer’s top priorities, and we believe this step is in the best interest of patients,” commented Aida Habtezion, Chief Medical Officer and Head of Worldwide Medical and Safety at Pfizer. "Our main concern lies with patients suffering from SCD, a severe and challenging-to-treat condition with limited therapies. We encourage patients to consult their doctors to explore alternative treatments as we continue to scrutinize the data findings."
Patients, doctors, pharmacists, or other healthcare professionals with further inquiries about OXBRYTA should contact Pfizer Medical Information at 1-800-438-1985. The company will keep patients, regulatory bodies, investigators, and clinicians updated on the actions and appropriate subsequent steps for OXBRYTA.
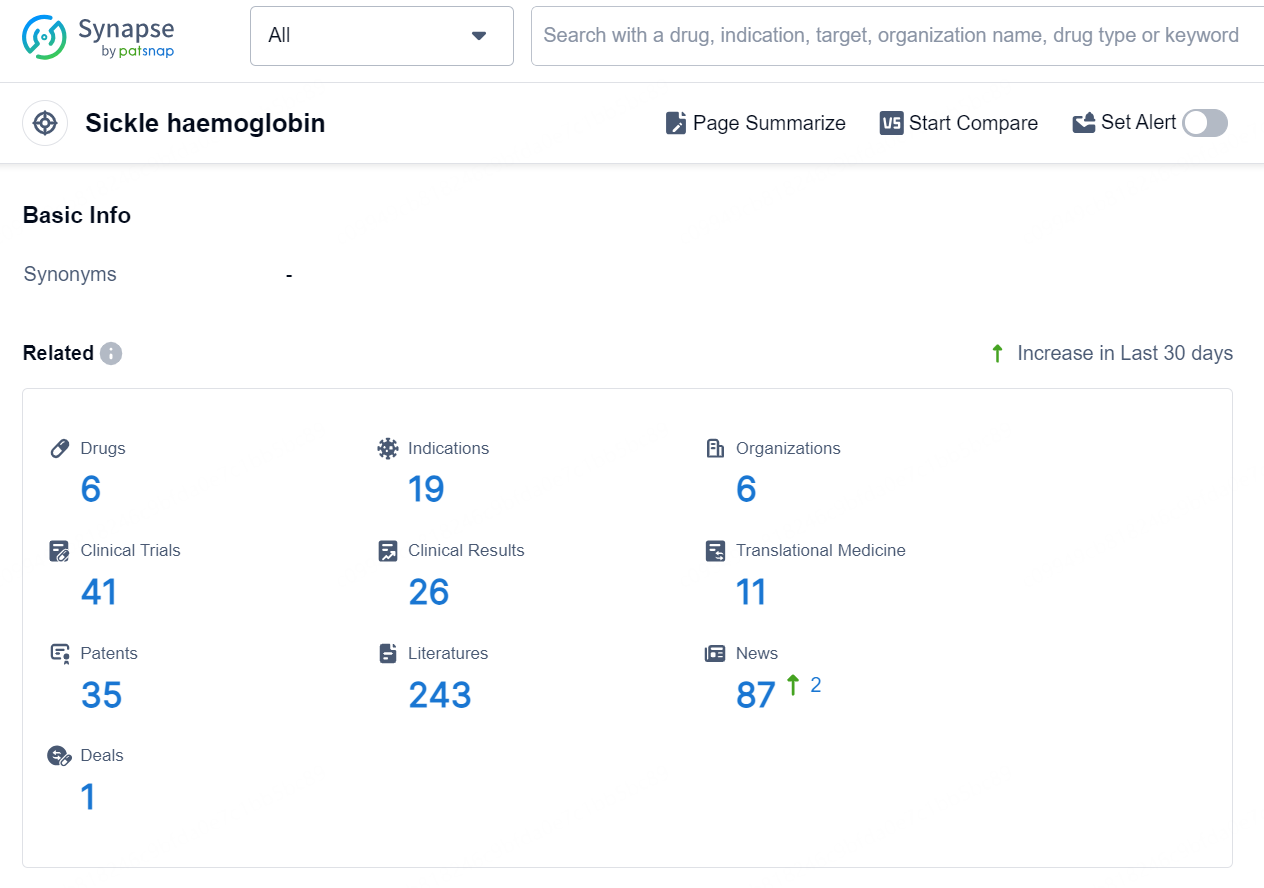
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of September 29, 2024, there are 6 investigational drugs for the sickle haemoglobin target, including 19 indications, 6 R&D institutions involved, with related clinical trials reaching 41, and as many as 35 patents.
Voxelotor is a small molecule drug developed by Global Blood Therapeutics, Inc. It was first approved in the United States in November 2019 for the treatment of anemia, specifically hemolytic anemia and sickle cell anemia. The drug targets sickle haemoglobin and falls into the therapeutic areas of congenital disorders, hemic and lymphatic diseases, and other diseases.