NMPA Approves Ivonescimab with Chemotherapy for EGFRm NSCLC in China: HARMONi-A Trial Highlights Improved Survival
Summit Therapeutics Inc. revealed that on May 24, 2024, their collaborator, Akeso Inc., obtained marketing approval in China from the National Medical Products Administration. This approval is grounded on favorable data from HARMONi-A, a single-region, multi-center, Phase III clinical trial executed in China, with data collection and analysis handled by Akeso.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The HARMONi-A study assessed the combination of ivonescimab and platinum-doublet chemotherapy in patients with epidermal growth factor receptor (EGFR)-mutated, locally advanced, or metastatic non-squamous non-small cell lung cancer (NSCLC) who have experienced progression after EGFR tyrosine kinase inhibitor therapy, comparing it to a placebo plus platinum-doublet chemotherapy.
This clinical investigation targets a patient demographic where Phase III global clinical trials with PD-1 monoclonal antibodies have previously been unsuccessful. The Phase III HARMONi-A trial adds to the growing body of evidence highlighting ivonescimab's unique mechanism of action. Ivonescimab, a PD-1/VEGF bispecific antibody, demonstrates cooperative binding properties.
This study and its data are distinct from those of the Phase III HARMONi-2 trial, which focuses on locally advanced or metastatic NSCLC patients with tumors expressing PD-L1 (PD-L1 TPS >1%). The current release is exclusively related to the HARMONi-A trial.
"We are thrilled to proceed with the accelerated development of ivonescimab, aiming to significantly benefit patients in need of innovative lung cancer therapies and treatments for other solid tumors," stated Dr. Maky Zanganeh, Chief Executive Officer and President of Summit.
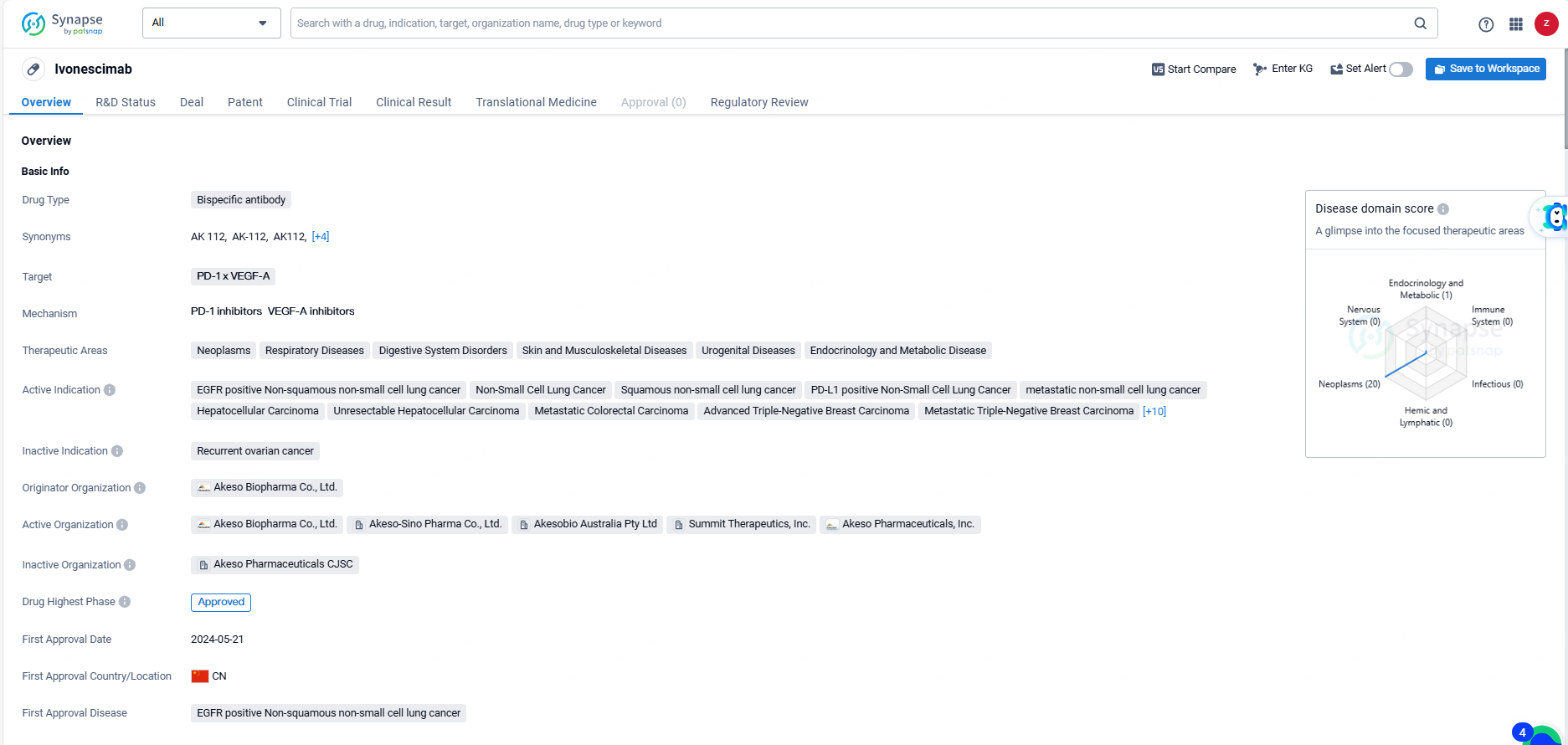
Ivonescimab is a bispecific antibody targeting PD-1 and VEGF-A, indicated for treating a broad spectrum of conditions including neoplasms, respiratory diseases, digestive system disorders, skin and musculoskeletal diseases, urogenital diseases, endocrinology, and metabolic disorders.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
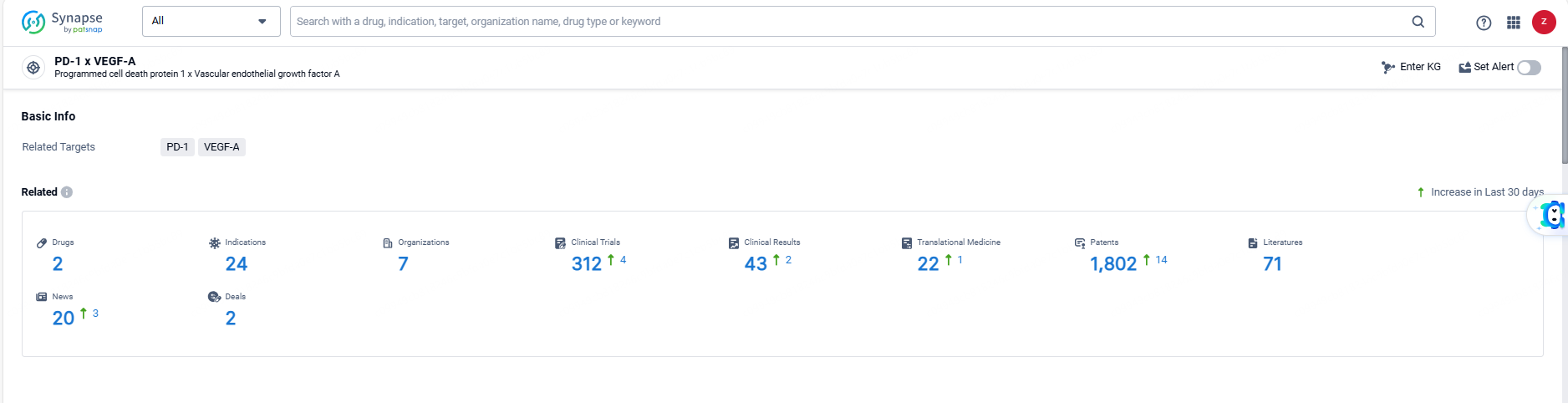
According to the data provided by the Synapse Database, As of June 6, 2024, there are 2 investigational drugs for the PD-1 and VEGF-A target, including 24 indications, 7 R&D institutions involved, with related clinical trials reaching 312, and as many as 1802 patents.
Ivonescimab is a bispecific antibody drug with a broad range of therapeutic indications, particularly in the treatment of various types of cancer. It has received approval in both global and Chinese markets, with a first approval date in China in 2024. The drug has been granted priority review and breakthrough therapy designation, indicating its potential to address unmet medical needs in the treatment of the specified therapeutic areas.