Ocular Therapeutix™ Initiates its Premier Vital Clinical Study for OTX-TKI
Ocular Therapeutix, Inc, a biotech firm specialising in the creation, advancement, and market deployment of novel treatments for eye diseases and disorders, has publicized the start of its premiere primary clinical trial to examine OTX-TKI, a proprietary axitinib intravitreal implant, for tackling wet age-related macular degeneration. Alongside, OTX-TKI's development for managing diabetic retinopathy and varied other retinal diseases is also underway.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"Ocular Therapeutix is excited to declare the commencement of our critical trial scrutinizing OTX-TKI's effectiveness in treating wet AMD. We anticipate collaborating with various clinical locations throughout America," expressed Antony Mattessich, CEO of Ocular Therapeutix. “This trial signifies a vital progression for our clinical scheme as we advance towards our objective of introducing a groundbreaking new therapy that can truly impact wet AMD patients grappling with vision loss."
The company has solicited a Special Protocol Assessment from the U.S. FDA, constructed as a superiority trial set to include around 300 assessable wet AMD patients who haven't received prior treatment in the study eye. The trial is devised as a multi-center, parallel-group trial chiefly conducted at U.S sites where patients will be allocated to receive either one injection of aflibercept or one OTX-TKI implant, succeeded by optional supplementary anti-VEGF treatment depending on particular criteria.
The effectiveness and safety of OTX-TKI will be determined by testing best corrected visual acuity and central subfield thickness at 36 weeks. While approval from the institutional review board has been obtained, the company plans not to recruit a subject until the SPA feedback is attained from the FDA.
Arshad M. Khanani, MD, MA, FASRS, Clinical Research Director at Sierra Eye Associates in Reno, Nevada, has consented to act as the chief investigator for the pivotal Phase 3 trial of OTX-TKI. Dr. Khanani is a globally acclaimed retina specialist with a breadth of knowledge in clinical trial construct for age-related macular degeneration treatment. He has taken on the role of a principal investigator for several clinical trials analyzing novel therapies for wet AMD and other retinal conditions.
OTX-TKI is a preliminary bioresorbable, hydrogel implant incorporating axitinib - a tiny molecule, multi-targeted, tyrosine kinase inhibitor with anti-angiogenic properties, under examination for treating wet age-related macular degeneration and other retinal diseases.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
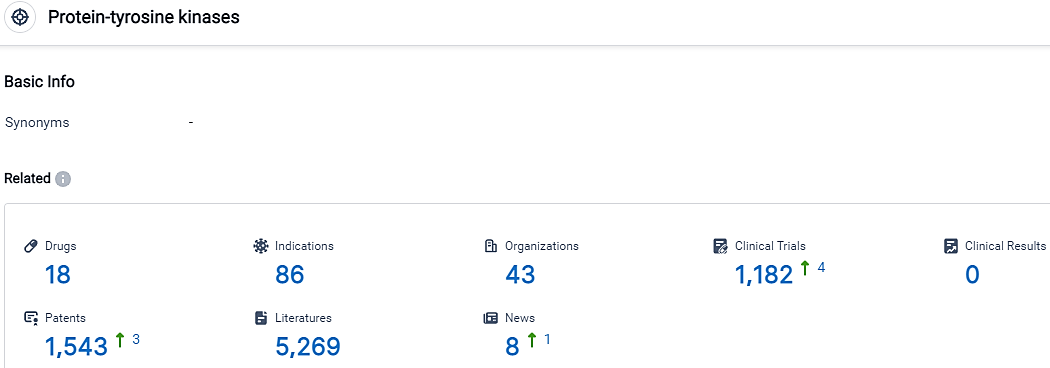
According to the data provided by the Synapse Database, As of October 11, 2023, there are 18 investigational drugs for the Protein-tyrosine kinases target, including 86 indications, 43 R&D institutions involved, with related clinical trials reaching 1182,and as many as 1543 patents.
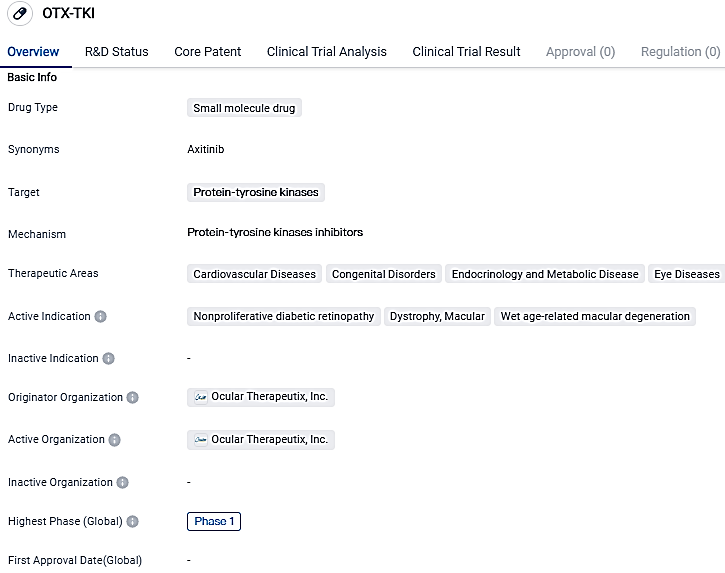
OTX-TKI is a small molecule drug targets protein-tyrosine kinases and shows promise in treating various cardiovascular, congenital, endocrine, and eye diseases. The drug is currently in Phase 1 of clinical development, where its safety, tolerability, and pharmacological properties are being evaluated.