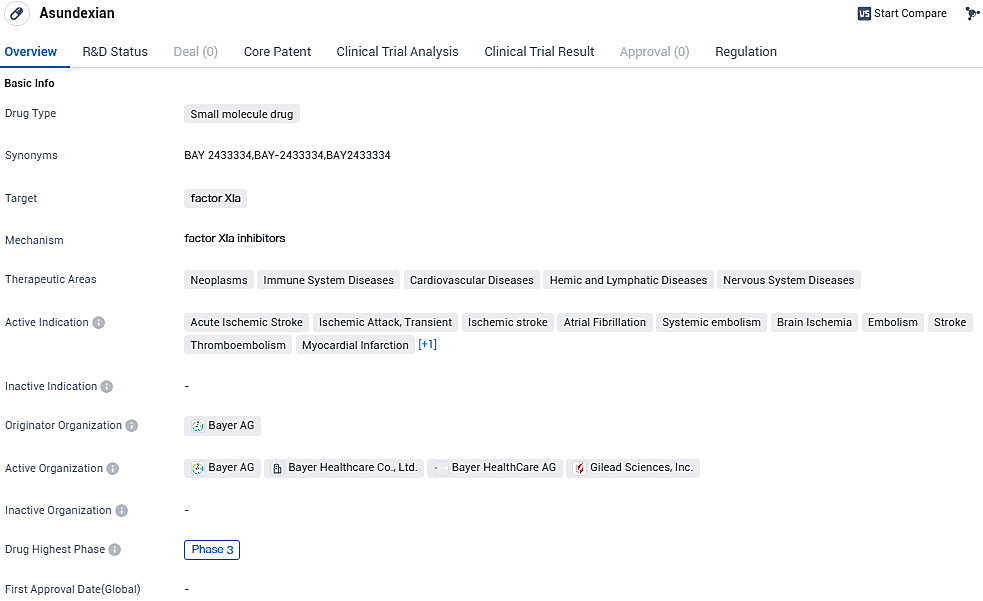
The OCEANIC-AF research was prematurely terminated due to insufficient effectiveness
The phase III trial dubbed OCEANIC-AF, which examines the effectiveness of asundexian against apixaban in stroke-prone atrial fibrillation patients, is set to terminate prematurely. This course of action follows the suggestion of the study's Independent Data Monitoring Committee during the consistent supervision, revealing that asundexian's efficacy is somewhat reduced in comparison to the control group. Bayer plans to undertake a deeper analysis of the results to comprehend the findings and will release the data afterwards.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Safety information available aligns well with previously recorded safety parameters for asundexian. The IDMC advises to proceed with the OCEANIC-STROKE phase III experiment as initially designed. Necessary steps will be taken to wind-up the OCEANIC-AF investigation and involved patients will be reached out by their respective physicians/investigators for discussing further course of action.
Despite the fact that the current analysis findings do not advocate for the continuation of the OCEANIC-AF trial, we remain committed to examining asundexian in the scope of the OCEANIC-STROKE research. "We are currently reassessing other potential uses in patients that require antithrombotic therapy," stated Dr. Christian Rommel, who is a part of Bayer AG's Pharmaceutical Division Executive Committee as well as the Global Head of Research and Development.
Additional proof implies the positive impact of anticoagulation treatment, added to the standard procedures, on the population represented in the OCEANIC-STROKE research that is in need of adequate therapy alternatives. Asundexian is an agent still being put through tests and it has not received approval from any heath regulatory for application in any country or for any indication.
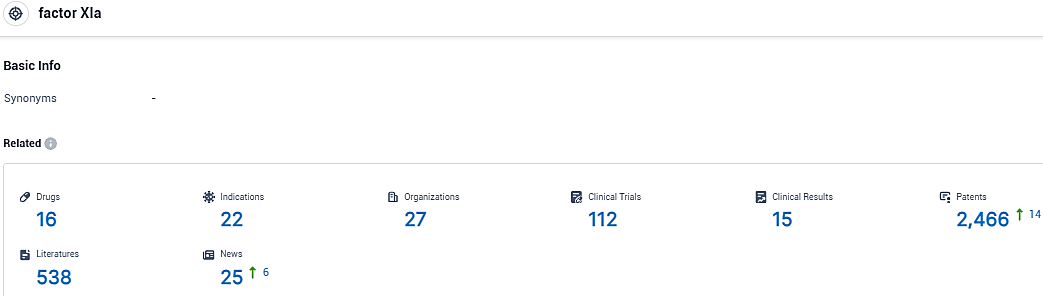
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 26, 2023, there are 16 investigational drugs for the factor XIa target, including 22 indications, 27 R&D institutions involved, with related clinical trials reaching 112, and as many as 2466 patents.
Asundexian, as an oral direct, potent inhibitor of activated coagulation factor XI (FXIa), acts selectively on the coagulation cascade, thereby offering the potential to prevent events like stroke without a corresponding increase in bleeding risk associated with the current standard of care. Asundexian is currently being evaluated as a potential treatment option in thrombosis prevention and could represent a new approach in antithrombotic treatment.