Onward Therapeutics Starts Recruiting for OT-A201 Dual-Targeted Antibody Clinical Study
Onward Therapeutics SA, an international biotech firm dedicated to creating cutting-edge immunotherapy solutions for oncology, has publicly stated that its phase 1 clinical study for OT-A201, a pioneering bispecific antibody that engages with two distinct immune checkpoints, is currently underway.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
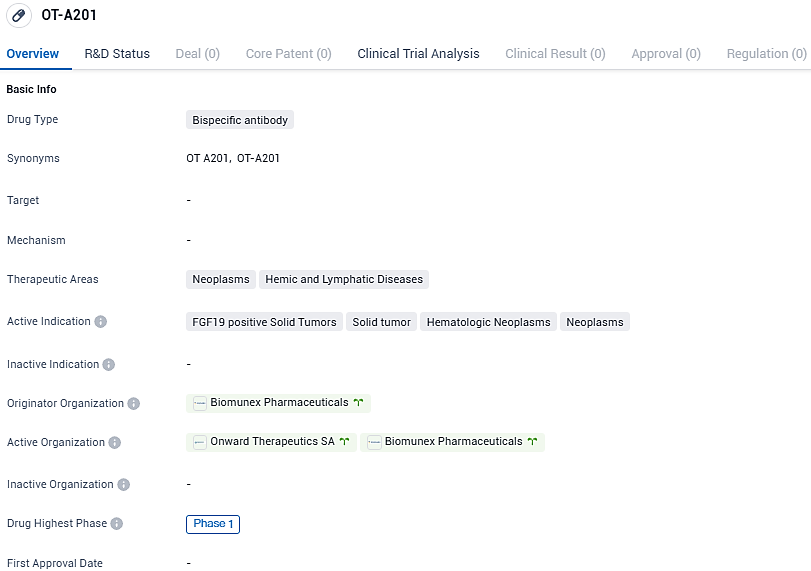
This research is focused on assessing the safety profile, tolerability, drug dynamics, immune response, and initial efficacy against cancer of the investigational drug OT-A201. It's a study taking place across multiple centers in Europe and features an open-label design, proceeding in two distinct phases.
The initial phase will involve escalating doses of OT-A201 administered solely to a group of patients suffering from re-emerged or stubborn blood cancers or those with widespread cancer that has spread to different parts of the body. The subsequent phase will aim to corroborate the safety and preliminary efficacy of OT-A201 used alone or in conjunction with other treatments, particularly focusing on certain blood cancers and solid tumors based on the dosage determined in the former phase.
The comprehensive global development, production, and sales rights for OT-A201 were obtained by Onward Therapeutics from Biomunex Pharmaceuticals. Both Onward Therapeutics and Biomunex are collaboratively advancing this dual-targeting antibody through the phase 1 clinical trial for at least one clinical indication. The commencement of treatment for the first patient has initiated a confidential milestone payment to Biomunex.
Dr. C. Grace Yeh, Chairman, and CEO has expressed, "Leveraging the drug development acumen and proven history of achievements of the Onward Therapeutics team, we've accelerated the progression from cellular line establishment, preclinical pharmacodynamics and safety evaluations, to clinical trials and regulatory submissions for OT-A201 with remarkable pace and efficiency. This trial signifies a crucial juncture for Onward Therapeutics as we evolve into a company immersed in clinical trials. Moreover, it underscores our commitment to the cultivation of pioneering ventures through our strategic 'buy-to-build' business approach."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
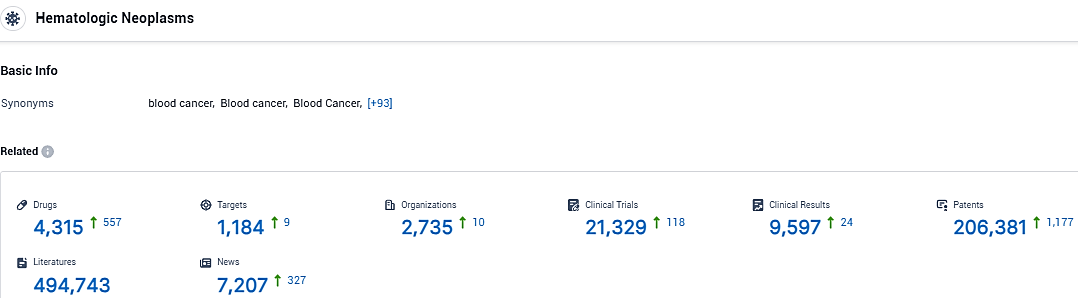
According to the data provided by the Synapse Database, As of January 29, 2024, there are 4315 investigational drugs for the Hematologic Neoplasms, including 1184 targets, 2735 R&D institutions involved, with related clinical trials reaching 9597, and as many as 206381 patents.
OT-A201 is a bispecific antibody drug being developed for the treatment of neoplasms and hemic and lymphatic diseases. It is currently in Phase 1 of clinical development and is being investigated for its potential in targeting FGF19 positive solid tumors, solid tumors, hematologic neoplasms, and neoplasms in general. Further research and clinical trials will be needed to determine the safety and efficacy of this drug.