Oscotec/ ADEL reveal FDA has granted the IND application clearance for ADEL-Y01 to treat Alzheimer's Disease
Oscotec Inc. and ADEL Inc. has announced that the FDA has given the IND application approval for ADEL-Y01 to be used in the treatment of Alzheimer's Disease.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
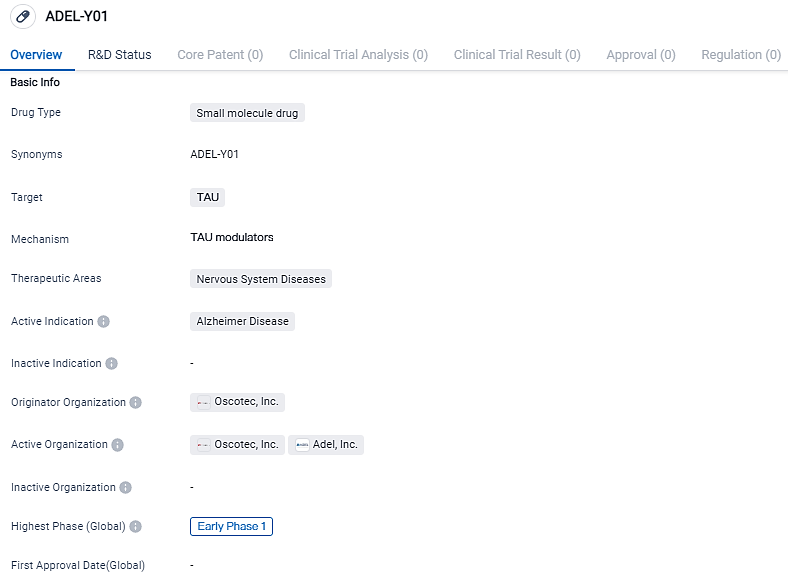
Oscotec and ADEL are collaboratively progressing a new immunotherapeutic method aimed at reducing tau protein level in the brain affected by AD. The therapeutic agent, ADEL-Y01, is a recombinant IgG1 class type monoclonal humanized antibody that identifies and attaches to the tau protein acetylated at lysine-280, thereby obstructing the aggregation and advancement of tau. It also enhances the disposal of tau by microglia.
In preclinical models, ADEL-Y01 treatment improved cognitive impairments, rectified behavioral deviations, and ameliorated tau pathology, which were published early this year in "Monoclonal antibody Y01 prevents tauopathy progression induced by lysine 280-acetylated tau in cell and mouse models" in The Journal of Clinical Investigation.
The objective of this Phase 1a/1b study is to interpret the safety, tolerability, pharmacokinetics, and clinical function of ADEL-Y01 both in healthy individuals and in participants showing Mild Cognitive Impairment resulting from Alzheimer's disease or mild Alzheimer's disease.
Dr. Taeyoung Yoon, CEO/CSO of Oscotec, commented, "We firmly believe that based on the unique mode of action and compelling preclinical results, ADEL-Y01 has the potential to emerge as a crucial treatment alternative for Alzheimer's patients."
Seung-Yong Yoon, M.D., CEO of ADEL, stated, "The IND approval by the FDA marks a significant milestone for us, notably as our initial candidate is transitioning into clinical trials. We eagerly look forward to initiating the clinical trial, as it takes us one step ahead towards delivering therapeutic interventions for patients struggling with tau-related disorders including AD."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
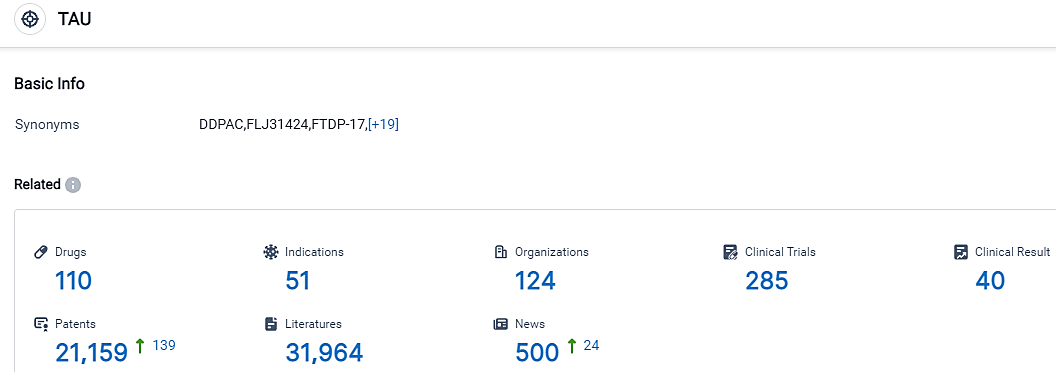
According to the data provided by the Synapse Database, As of September 18, 2023, there are 110 investigational drugs for the TAU target, including 51 indications,124 R&D institutions involved, with related clinical trials reaching 285,and as many as 21159 patents.
ADEL-Y01 is a small molecule drug for the treatment of Alzheimer's Disease. It targets TAU, a protein associated with the disease, and is currently in the early phase 1 of clinical development. Further studies are needed to evaluate its potential as a therapeutic option for Alzheimer's patients.