Nektar Therapeutics Reports Encouraging Preliminary Results from Phase 1b Trial of Rezpegaldesleukin
Nektar Therapeutics disclosed fresh clinical results concerning rezpegaldesleukin utilised for patients battling atopic dermatitis, featuring new clinical effectiveness parameters from its Phase 1b research. Rezpegaldesleukin represents Nektar's innovative, unprecedented selective regulatory T-cell therapy, currently under development for managing atopic dermatitis.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Nektar Therapeutics is showcasing the fresh Phase 1b clinical efficacy endpoints for rezpegaldesleukin. The latest data underscore the significant potential of rezpegaldesleukin in providing relief to those suffering from atopic dermatitis, a skin disorder affecting roughly 10% of the US population. Furthermore, details concerning the trial formats for their upcoming Phase 2b study of rezpegaldesleukin in atopic dermatitis, kicking off in October, along with a newly proposed Phase 2a study commencing early 2024 in alopecia areata will also be shared.
"Today's release of new findings relating to rezpegaldesleukin in atopic dermatitis confirms rezpegaldesleukin's potential as a unique therapeutic that could significantly enhance the lives of patients. This is besides the powerful efficacy already reported for EASI-related endpoints," commented Nektar Therapeutics' Chief R&D Officer, Jonathan Zalevsky, Ph.D.
"We eagerly anticipate proceeding with rezpegaldesleukin into our comprehensive Phase 2b study involving biologic-naïve patients living with moderate to severe atopic dermatitis by October this year. We're thrilled to also share news of a Phase 2a study commencing in 2024, set to explore the use of rezpegaldesleukin in treating patients with alopecia areata," Zalevsky added.
The double-blind, placebo-controlled Phase 1b study on rezpegaldesleukin's application in atopic dermatitis was conducted to determine its safety, tolerance, and pharmacokinetics over a 12-week induction treatment period. Patients who met the ≥EASI-50 response at Week 19 were monitored for an additional 36 weeks post-treatment or until they no longer met the EASI-25 response criteria. The initial study analyzed 44 patients with moderate-to-severe AtD who had not responded to topical corticosteroids.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
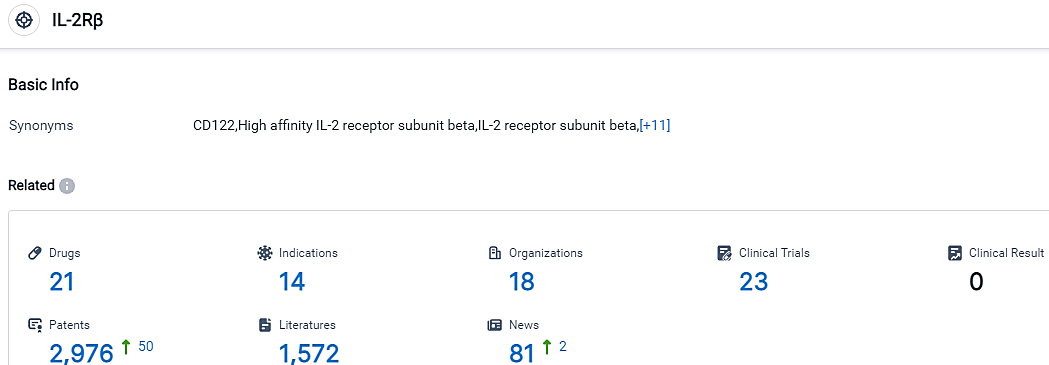
According to the data provided by the Synapse Database, As of September 18, 2023, there are 21 investigational drugs for the IL-2Rβ target, including 14 indications,18 R&D institutions involved, with related clinical trials reaching 23,and as many as 2976 patents.
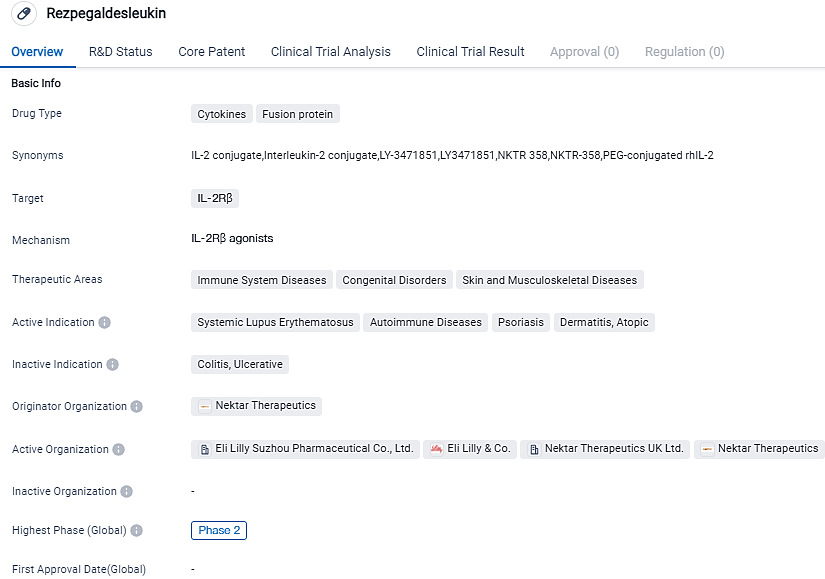
Rezpegaldesleukin is a cytokine fusion protein drug that targets IL-2Rβ. It is being developed for the treatment of immune system diseases, congenital disorders, and skin and musculoskeletal diseases. The drug has reached Phase 2 of clinical development globally and is currently at the IND application stage in China.