Coeptis Therapeutics provides update on DVX201's Phase 1 Trials safety and dosage for Relapsed/Refractory AML or High Risk MDS patients
Coeptis Therapeutics Holdings, Inc. a firm specializing in biopharmaceutical research and pioneering cell-based therapies for cancer, shared patient dosing and safety information from two Phase 1 clinical studies. These trials examine DVX201 as a potential treatment for those suffering from either relapsed/refractory acute myeloid leukemia or high-risk myelodysplastic syndrome, as well as infected COVID-19 patients needing hospitalization.
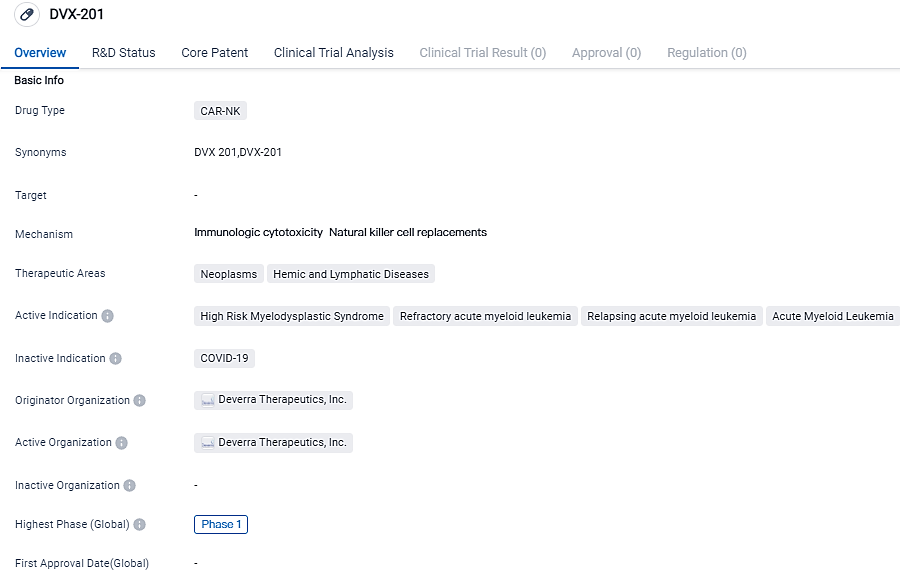
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
DVX201, an innovative allogeneic and unaltered natural killer cell treatment, is created using CD34+ hematopoietic stem and progenitor cells derived from a collection of donors. Preliminary results from two clinical trials, which consisted of 16 patients and 23 infusions of DVX201, suggest that the NK cell therapy is well-accepted with no dosage-related toxic effects, cytokine discharge disease, or infusion-related toxicities observed up to the highest dosage level.
A Phase 1 clinical trial assessing DVX201 for patients with hospitalised COVID-19 infection has successfully finished three dosing cohorts, with a total of nine patients each receiving one infusion. DVX201 has been well-received at all dosage levels.
For the Phase 1 trial of DVX201 in relapsed/refractory AML or high-risk MDS, seven test subjects, each receiving two infusions, have been safely administered. The trial anticipates to admit three to five more patients who will be treated at the highest dosage level. By the first quarter of 2024, Coeptis believes it will disclose preliminary safety and efficiency outcomes from the full patient sample.
Colleen Delaney, MD, Chief Scientific and Medical Officer, said "DVX201, a pooled donor product, signifies a genuinely innovative manufacturing platform. The current safety findings give us optimism as we persist in admitting more subjects into the highest dosage cohort for the remaining part of the Phase 1 trial for relapsed/refractory AML and high-risk MDS, which should amount to seven subjects for 14 infusions. We forecast to receive primary data for this trial within the first quarter of 2024."
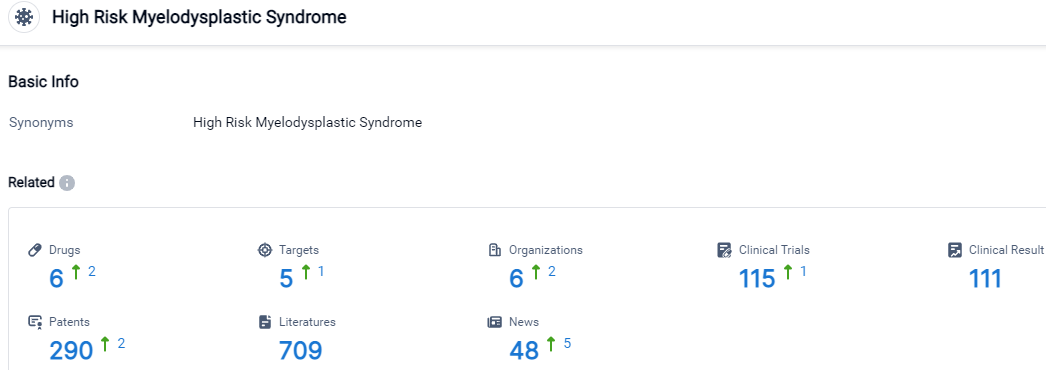
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, target, organizations, clinical trials, clinical results, and drug patents related to this indications.
According to the data provided by the Synapse Database, As of September 18, 2023, there are 6 investigational drugs for the High Risk Myelodysplastic Syndrome, including 5 target, 6 R&D institutions involved, with related clinical trials reaching 115,and as many as 290 patents.
DVX-201 is a CAR-NK drug for the treatment of various types of leukemia, including High Risk Myelodysplastic Syndrome and Acute Myeloid Leukemia. The drug is currently in Phase 1 of clinical trials, and further research is required to determine its potential as a therapeutic option for patients with these conditions.