Otsuka and Astex declare the European Commission's authorization of INAQOVI® (oral decitabine and cedazuridine) to treat myeloid leukaemia
Otsuka Pharmaceutical Europe Ltd. along with Astex Pharmaceuticals, Inc. have revealed that INAQOVI®(oral decitabine and cedazuridine) has received approval by the EC to be used as a solo treatment for newly identified acute myeloid leukaemia in adult patients who are unfit for the conventional induction chemotherapy.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The EC decision applies to the European Economic Area, encompassing EU member countries, Iceland, Liechtenstein, and Norway. The first and only orally administered hypomethylating agent licensed for use in the EEA on this particular group of patients is INAQOVI®.
The EC approval is grounded on data from the ASCERTAIN Phase 3 clinical trial, which assessed the equivalence of pharmacokinetic exposure of the groundbreaking oral fixed-dose mixture against intravenous decitabine in patients suffering from AML.
The ASCERTAIN investigation accomplished its main objective, confirming the pharmacokinetic exposure equality of the orally taken decitabine and cedazuridine fixed-dose combination compared to a standard five-day course of IV decitabine, based on a two-cycle, cross-over research design. The safety outcomes of the fixed-dose combination of decitabine and cedazuridine generally aligned with those projected for IV decitabine.
The current treatment alternatives for AML-affected adults span from IV chemotherapy infusions managed at healthcare facilities to, for patients ineligible for chemotherapy, treatment plans grounded on parenterally administered hypomethylating agents, normally stretching for 5-7 days. Fatigue may substantially hamper daily routine and affect a patient's life quality. INAQOVI® could offer both patients and medical practitioners an oral treatment choice in this patient group.
On 10th June 2022, the EMA agreed to a Paediatric Investigation Plan for the oral decitabine and cedazuridine fixed-dose combination, representing an important milestone for the prospect of furthering clinical studies in children with AML.
On June 10, 2022, the EMA agreed to a Paediatric Investigation Plan for the oral fixed-dose combination of decitabine and cedazuridine. This signifies a substantial progress towards the potential of advancing clinical trials in pediatric patients with AML.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
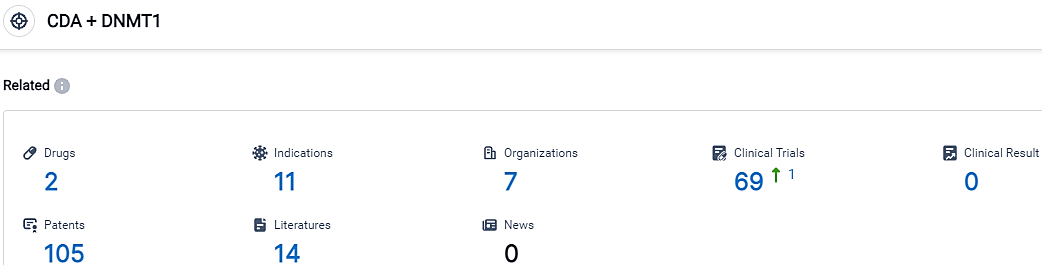
According to the data provided by the Synapse Database, As of September 22, 2023, there are 2 investigational drugs for the CDA and DNMT1 target, including 11 indications,7 R&D institutions involved, with related clinical trials reaching 69,and as many as 105 patents.
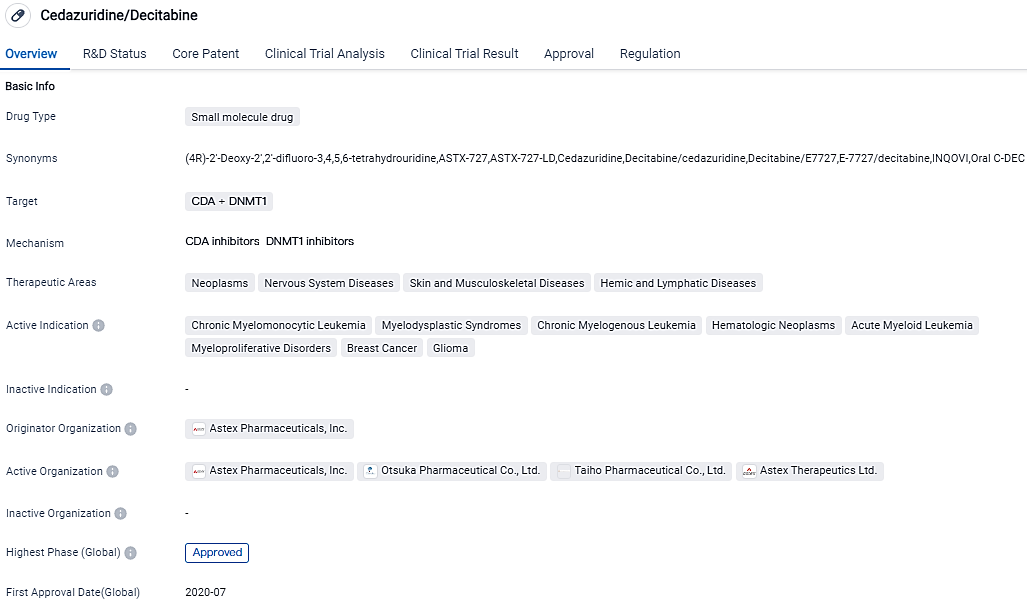
Cedazuridine/Decitabine targets CDA and DNMT1 and is used in the treatment of various neoplasms, nervous system diseases, skin and musculoskeletal diseases, and hemic and lymphatic diseases. It has been approved for use in several indications, including Chronic Myelomonocytic Leukemia, Myelodysplastic Syndromes, Chronic Myelogenous Leukemia, Hematologic Neoplasms, Acute Myeloid Leukemia, Myeloproliferative Disorders, Breast Cancer, and Glioma. Its first approval was in July 2020 in the United States and Canada.