Overview of Arvinas’s Drug Pipeline | R&D Progress | Drug Target
Arvinas, Inc. is a biopharmaceutical company that was founded in 2013 and is based in Connecticut, United States. The company focuses on developing innovative therapies in the field of biomedicine. Arvinas is committed to improving the lives of patients with debilitating and life-threatening diseases by discovering, developing and commercializing therapies that reduce disease-causing proteins.
The company uses Protac Discovery Engine, a proprietary technology platform, to design chimera-targeted protein breakdowns, or targeted protein degraders, designed to selectively remove disease-causing proteins using the body's own natural protein disposal system. The Company's small molecule PROTAC technology has the potential to address a wide range of intracellular disease targets, including those that represent up to 80% of proteins, and the Company is using the Protac Discovery Engine to build a broad pipeline of protein degradation product candidates to target diseases in oncology (including immuno-oncology), neuroscience, and other therapeutic areas. In this report, we will analyze the distribution of therapeutic areas, the most frequently developed targets, and the pipeline of Arvinas, Inc.
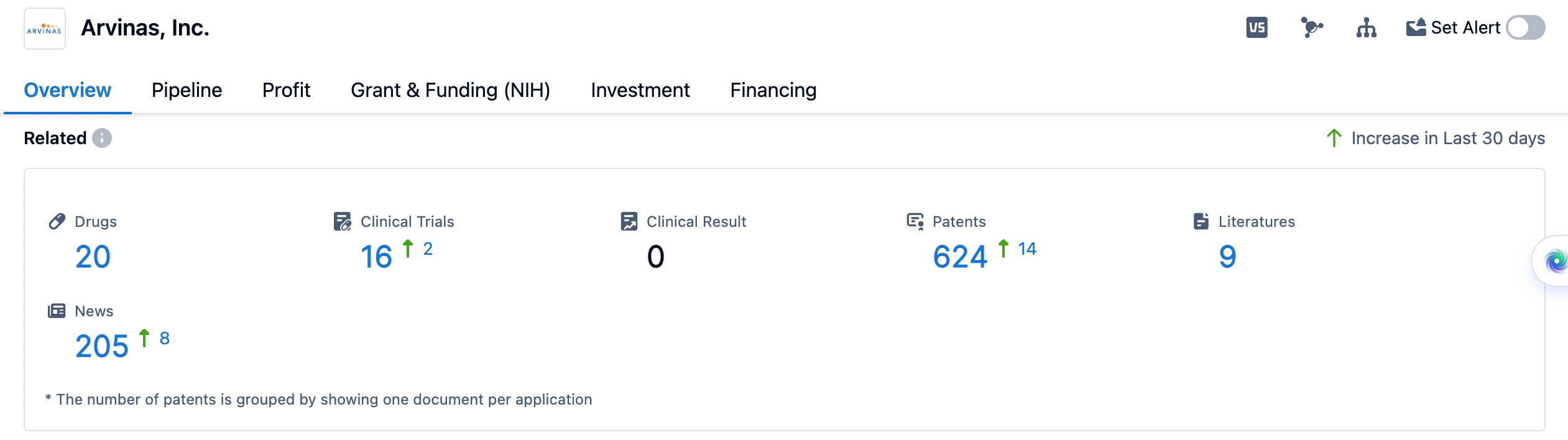
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of Arvinas.
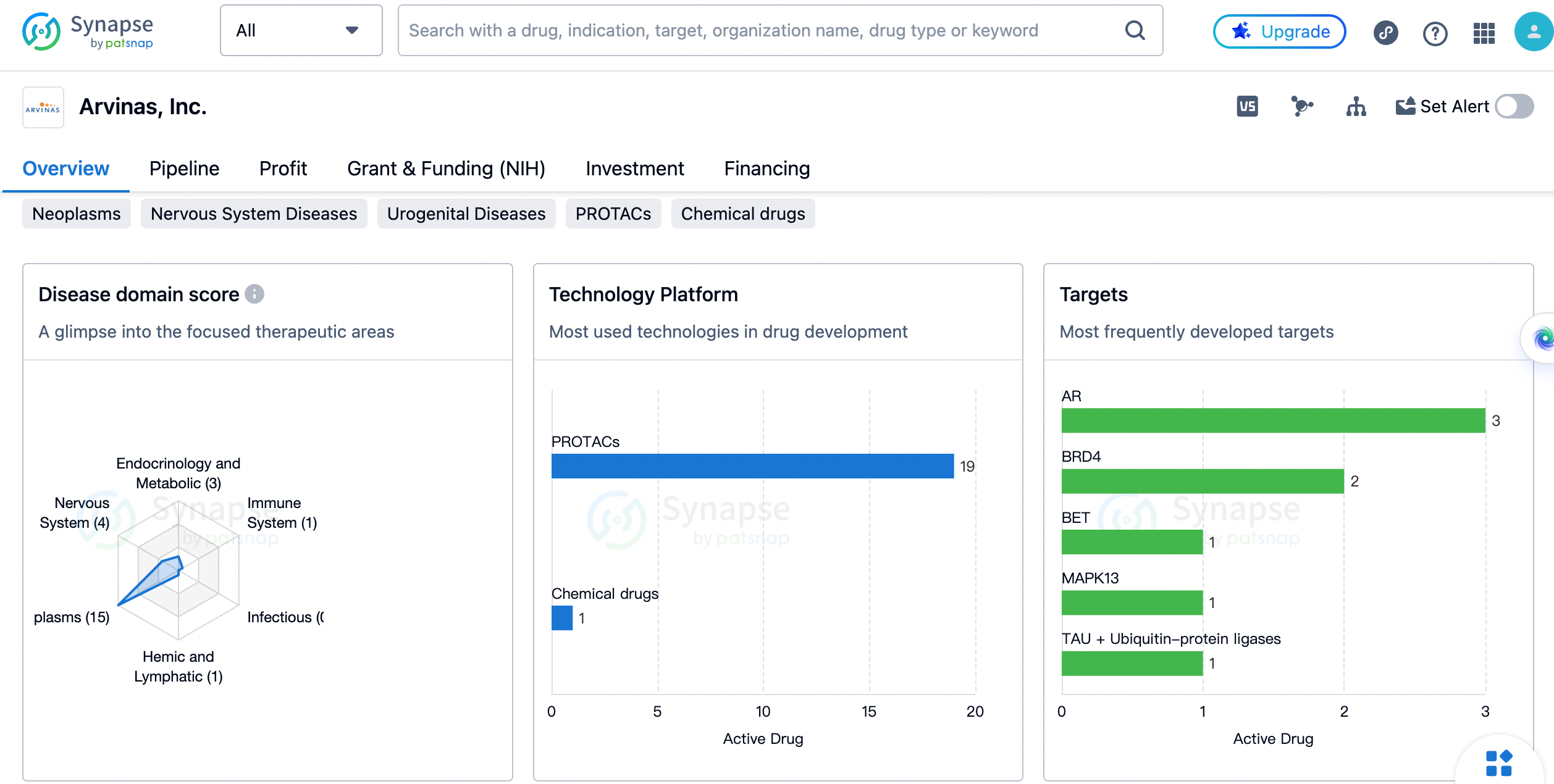
The distribution of therapeutic areas
The company has the highest number of drugs in the therapeutic area of Neoplasms, with a count of 15. This indicates that Arvinas, Inc. has a strong focus on developing therapies for cancer treatment. Nervous System Diseases and Urogenital Diseases are the next two therapeutic areas with 4 drugs each. This suggests that the company is also actively involved in developing treatments for neurological and urogenital disorders.
The most frequently developed targets by Arvinas
The androgen receptor (AR) is the most targeted protein, with 3 drugs developed by the company. This suggests that Arvinas, Inc. is focusing on developing therapies that target AR-related diseases, such as prostate cancer. BRD4 is the second most targeted protein, with 2 drugs developed. Other targets, such as BET, MAPK13, TAU + Ubiquitin-protein ligases, c-Myc, AR-v7, α-synuclein, FLT3, HTT, HPK1, ERα, KRAS G12D + KRAS G12V, SMARCA2, and BCL6, have one drug each developed by Arvinas, Inc. This indicates that the company has a diverse range of targets in its drug development pipeline.
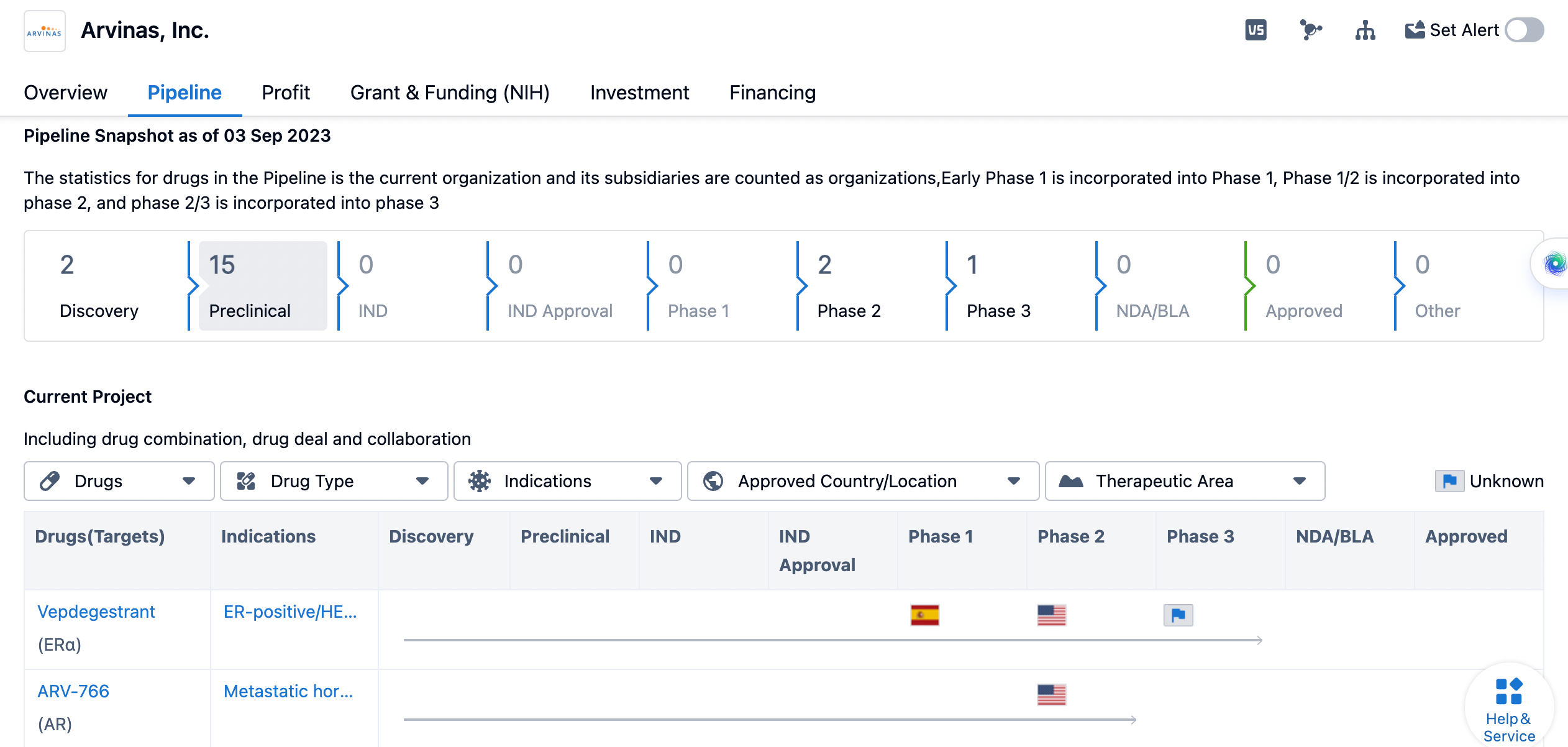
overview of Arvinas's pipeline status
The company has 2 drugs in the discovery phase, indicating ongoing research and development activities. There are 15 drugs in the preclinical phase, suggesting that Arvinas, Inc. is actively conducting preclinical studies to evaluate the safety and efficacy of these drug candidates. However, there are no drugs in the IND (Investigational New Drug) phase or IND approval phase. In terms of clinical development, Arvinas, Inc. has 2 drugs in Phase 2 and 1 drug in Phase 3. This suggests that the company has progressed some of its drug candidates to advanced stages of clinical trials, indicating promising results in earlier phases.
In summary, Arvinas, Inc. is a biopharmaceutical company that was founded in 2013 and is based in Connecticut, United States. The company has a strong focus on developing therapies for neoplasms, with the highest number of drugs in this therapeutic area. Arvinas, Inc. also has a significant presence in the development of therapies for nervous system diseases and urogenital diseases. The company targets a diverse range of proteins, with the androgen receptor being the most frequently targeted. Arvinas, Inc. has a pipeline of drugs in various stages of development, with a significant number of drugs in the preclinical phase. The company has progressed some of its drug candidates to Phase 2 and Phase 3 clinical trials, indicating promising results.