A Comprehensive Review of Drotaverine Hydrochloride's R&D Innovations
Drotaverine Hydrochloride's R&D Progress
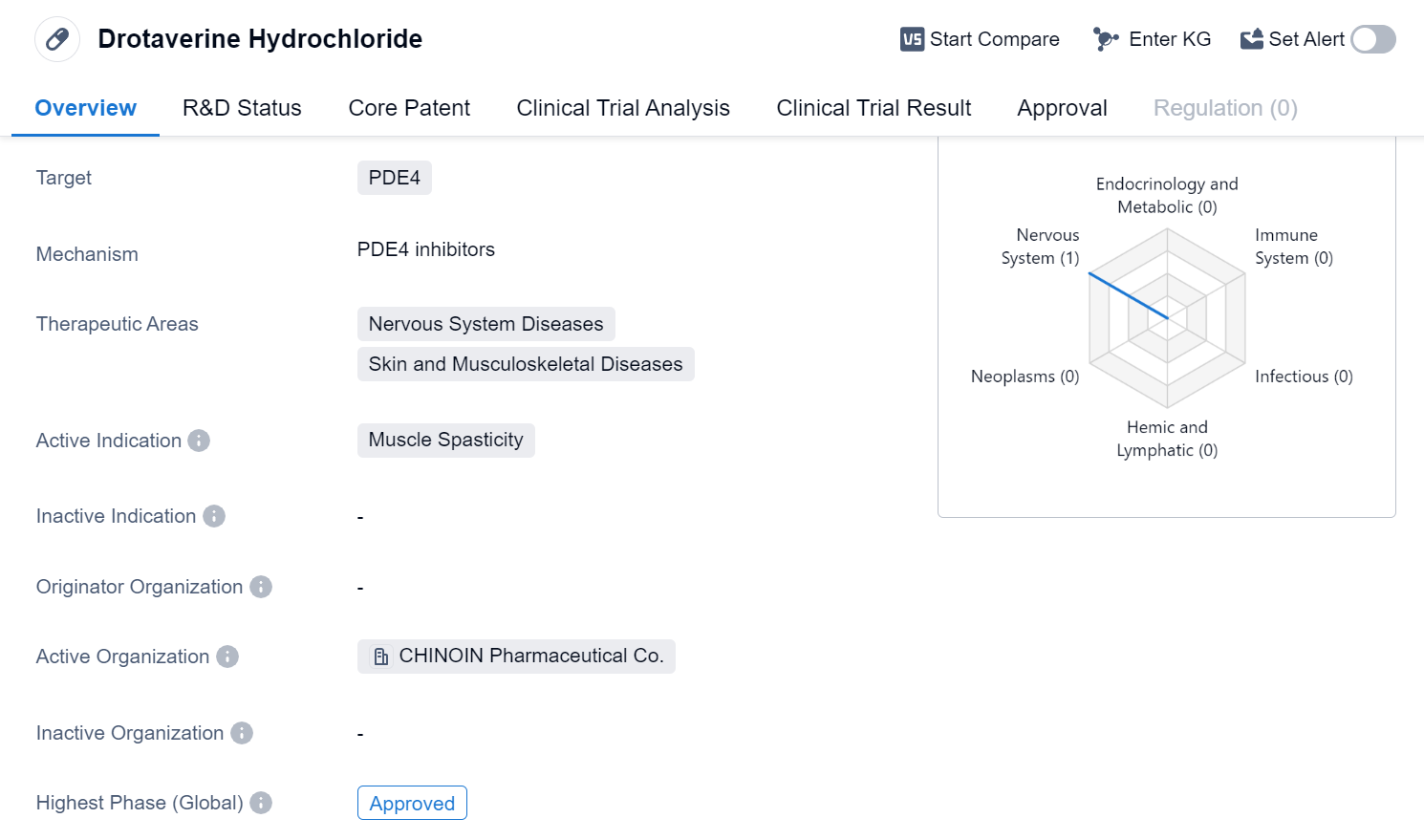
Drotaverine Hydrochloride is a small molecule drug that primarily targets PDE4, an enzyme involved in various cellular processes. This drug is primarily used in the treatment of muscle spasticity, a condition characterized by involuntary muscle contractions and stiffness. Muscle spasticity can occur in various diseases and conditions affecting the nervous system, skin, and musculoskeletal system.
Drotaverine Hydrochloride has successfully completed clinical trials and has been approved for use in global market.
Muscle spasticity can be a debilitating condition that significantly affects a patient's quality of life. It can cause pain, limited mobility, and functional impairment. Drotaverine Hydrochloride offers a potential solution for managing muscle spasticity and improving patient outcomes.
As a small molecule drug, Drotaverine Hydrochloride is likely to have a well-defined chemical structure and can be easily synthesized in a laboratory setting. This characteristic makes it suitable for large-scale production and distribution, ensuring its availability to patients in need.
The therapeutic areas of nervous system diseases, skin diseases, and musculoskeletal diseases highlight the potential broader applications of Drotaverine Hydrochloride beyond muscle spasticity. It suggests that this drug may have additional benefits in treating other conditions within these therapeutic areas. However, further research and clinical trials may be required to explore these potential indications.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Drotaverine Hydrochloride: PDE4 inhibitors
PDE4 inhibitors are a type of medication that target and inhibit the enzyme phosphodiesterase 4 (PDE4). Phosphodiesterase enzymes are responsible for breaking down cyclic adenosine monophosphate (cAMP), which is an important signaling molecule involved in various cellular processes. By inhibiting PDE4, these inhibitors increase the levels of cAMP, leading to a variety of effects.
From a biomedical perspective, PDE4 inhibitors have shown potential in the treatment of various diseases, particularly inflammatory conditions such as asthma, chronic obstructive pulmonary disease (COPD), and psoriasis. By increasing cAMP levels, PDE4 inhibitors can reduce inflammation and suppress the immune response, thereby alleviating symptoms associated with these conditions.
In the context of biomedicine, PDE4 inhibitors are considered a promising therapeutic approach due to their ability to modulate inflammatory pathways. However, it is important to note that these inhibitors may also have side effects, such as gastrointestinal disturbances, headache, and nausea. Therefore, careful consideration and monitoring are necessary when using PDE4 inhibitors as a treatment option.
Drug Target R&D Trends for Drotaverine Hydrochloride
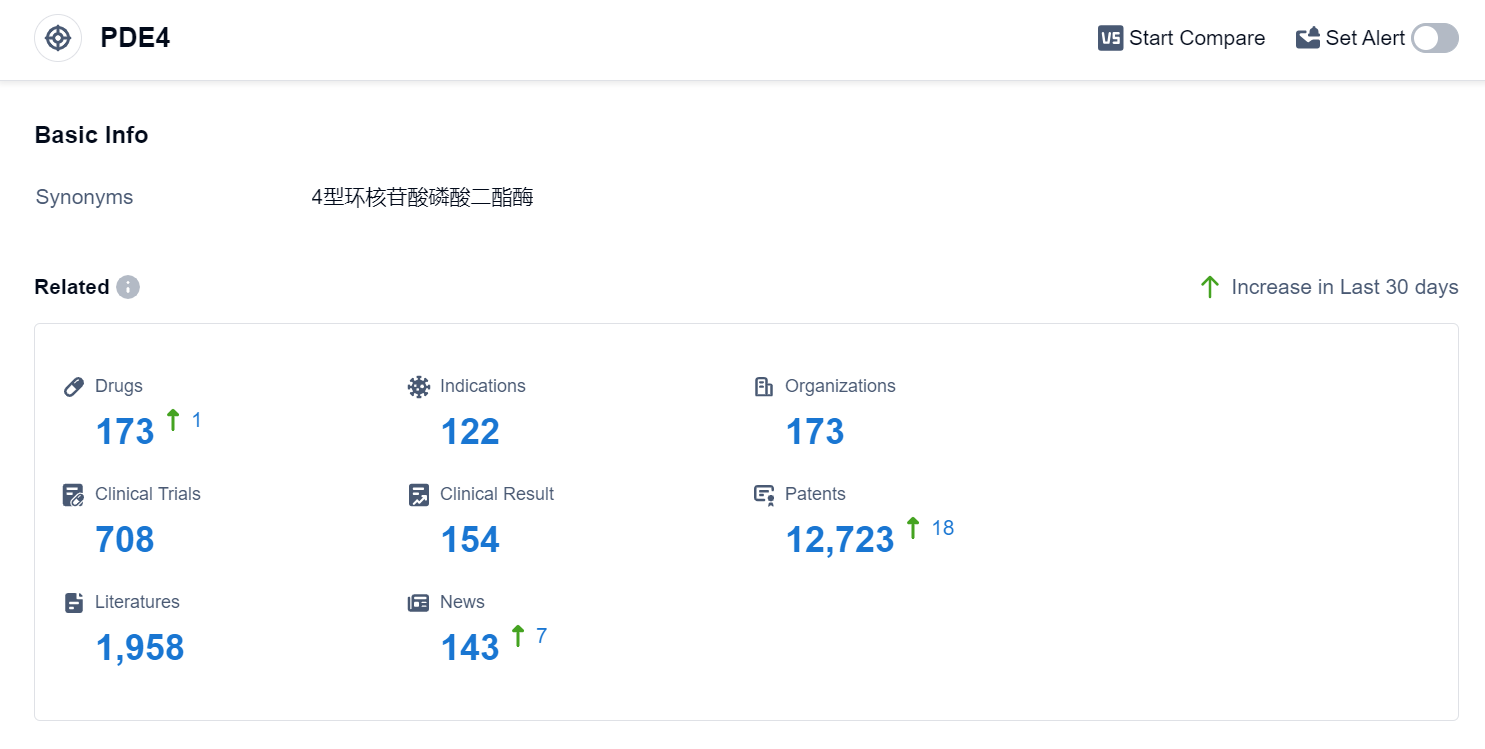
According to Patsnap Synapse, as of 4 Sep 2023, there are a total of 173 PDE4 drugs worldwide, from 173 organizations, covering 122 indications, and conducting 708 clinical trials.
The analysis of the current competitive landscape of target PDE4 reveals that Pfizer Inc., Bristol Myers Squibb Co., and Takeda Pharmaceutical Co., Ltd. are the leading companies in terms of R&D progress. Drugs targeting PDE4 have been approved for various indications, indicating their potential in treating a wide range of diseases. Small molecule drugs are progressing rapidly, but there is intense competition in this space. China, the United States, and the European Union are the countries/locations with the fastest development, with China showing significant progress in PDE4 research and development. The future development of target PDE4 is promising, with ongoing research and development efforts by multiple companies and countries.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In conclusion, Drotaverine Hydrochloride is a small molecule drug that targets PDE4 and is primarily used for the treatment of muscle spasticity. It has successfully completed clinical trials and has been approved for use in global market. This drug offers a potential solution for managing muscle spasticity and improving patient outcomes. Its therapeutic areas indicate potential broader applications in nervous system diseases, skin diseases, and musculoskeletal diseases. However, further research is needed to explore these potential indications.