PeproMene Bio Reports Complete Remission for Initial Patient in B-ALL Study with PMB-CT01 at City of Hope
PeproMene Bio, Inc., a firm focused on creating innovative treatments in the clinical-stage biotechnological space for cancer and immune diseases, has reported that the first participant from the initial group in its Phase 1 study on recurring or resistant B-cell acute lymphoblastic leukemia, using PMB-CT01 (BAFFR-CAR T Cells), has achieved a complete remission one month following treatment.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
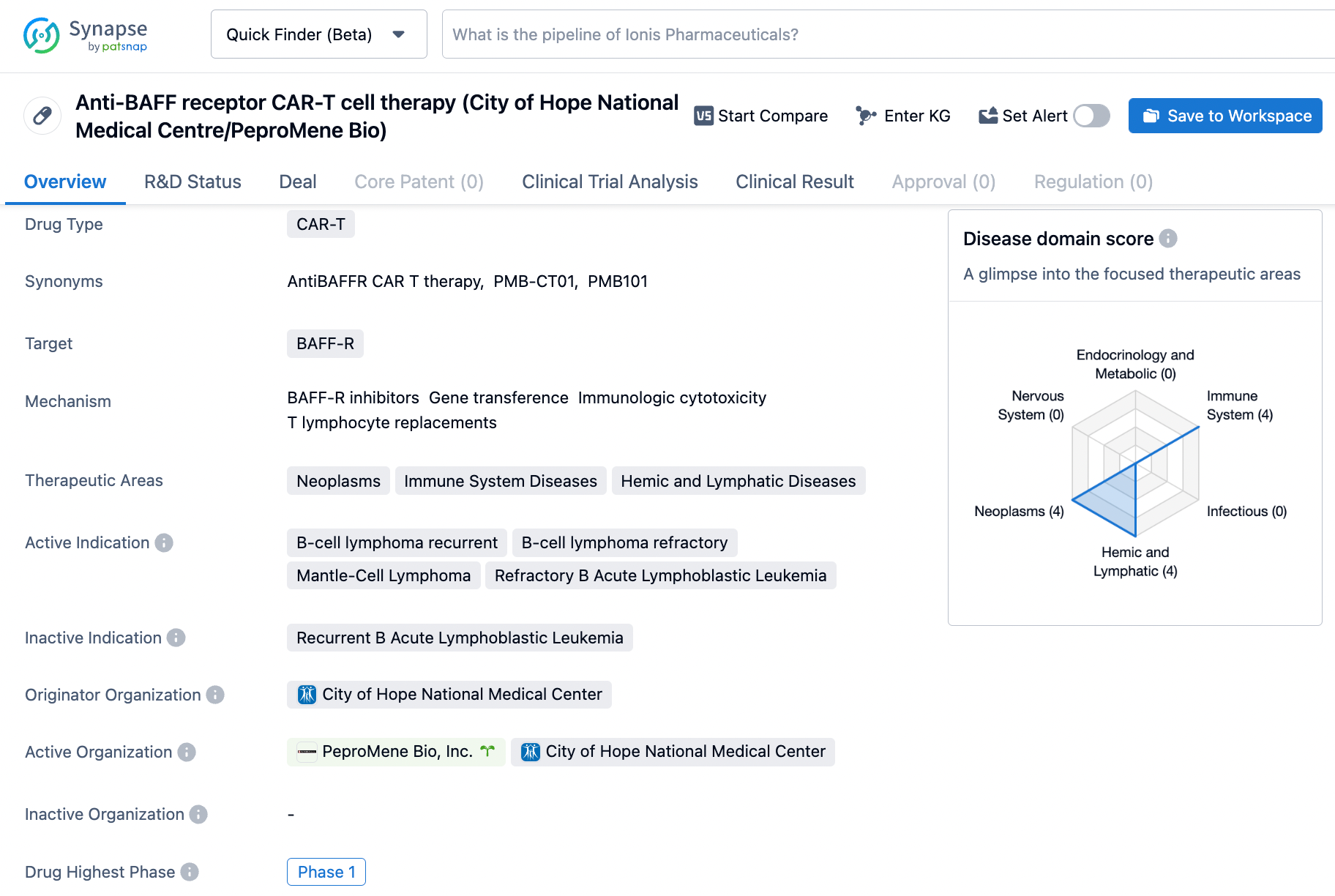
The conclusions of PeproMene Bio's clinical trial PMB-CT01 for recurrent/refractory non-Hodgkin's lymphoma was revealed at previous month's assembly of the American Society of Hematology in San Diego. Elizabeth Budde, MD, PhD, City of Hope associate professor in Hematology & Hematopoietic Cell Transplantation, Division of Lymphoma, instigated great interest by showing the trial outcomes in which all three subjects achieved long-lasting total remissions with minor side effects.
The studies are being carried out at City of Hope, which ranks among the major cancer researchers and treatment providers in the United States, and is also the creator of this therapy. For the initial month of therapy, the patient reported only milder treatment-related adverse effects, including a grade 1 cytokine release syndrome that resolved without intervention and no case of immune effector cell-associated neurotoxicity syndrome.
Ibrahim T. Aldoss, MD, an associate professor at City of Hope's Department of Hematology & Hematopoietic Cell Transplantation and the lead researcher of this single-center, dose escalation trial with expansion, stated, "The remarkably good results of this treatment for a patient suffering from a B-cell ALL relapse following chemotherapy and blinatumomab treatment is cause for optimism. His recurring disease was CD19- and CD22-negative, which suggests that there were significantly scant treatment options available for him."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
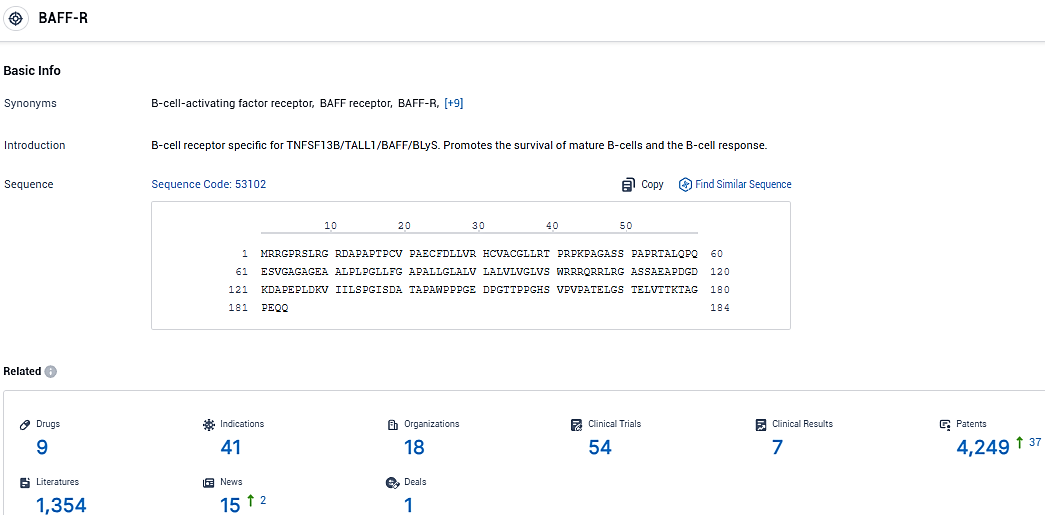
According to the data provided by the Synapse Database, As of January 24, 2024, there are 9 investigational drugs for the BAFF-R target, including 41 indications, 18 R&D institutions involved, with related clinical trials reaching 54, and as many as 4249 patents.
PMB-CT01 is a first-in-class BAFFR-targeted, autologous CAR T Cell therapy. By targeting the BAFFR protein, the drug aims to disrupt the growth and survival of cancer cells. It is currently in Phase 1 of clinical development, indicating its early stage of testing in humans. The drug shows potential in addressing the unmet medical needs in B-cell lymphoma and refractory B Acute Lymphoblastic Leukemia.