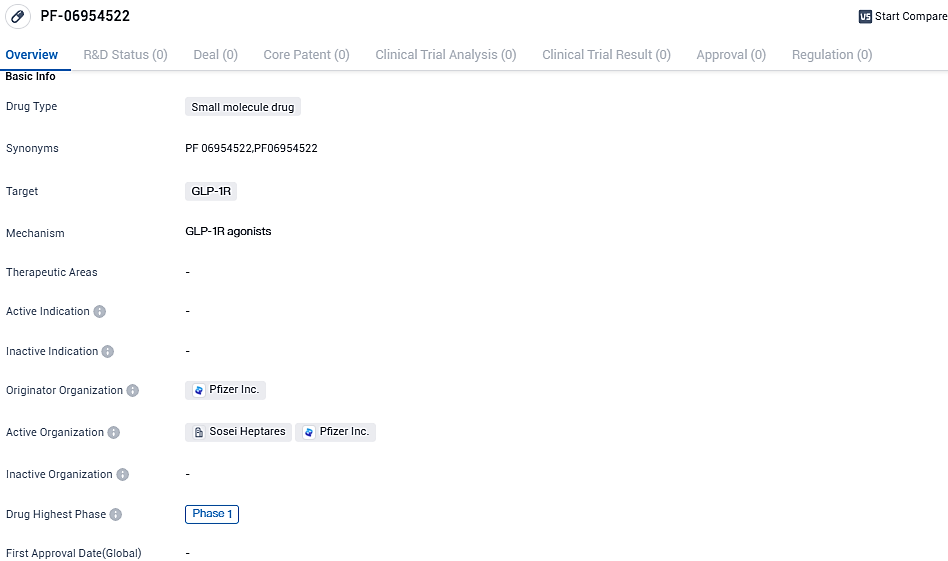
Pfizer, Sosei Heptares’ collaborator, moves forward with the Phase 1 trial of its GLP-1 receptor agonist PF-06954522
Sosei Group Corporation was informed by Pfizer that it had initiated a Phase 1 clinical trial with a new oral small molecule GLP-1 receptor agonist. This new drug, dubbed PF-06954522, was uncovered during a joint research venture with multiple targets by Pfizer scientists. Sosei Heptares' exclusive StaR® (stabilized receptor) technology was used throughout this process. Pfizer made public the introduction of PF-06954522 into its internal medicine-centric clinical development pathway as part of its third-quarter 2023 updates on October 31, 2023.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Dr. Matt Barnes, the leader of Heptares Therapeutics and chief of research in the UK, has shared his excitement about the progression of PF-06954522 into clinical trials, a result of the concerted research efforts with Pfizer. This advances the possibility of new medications to treat metabolic diseases that require more medical solutions.
In 2015, Pfizer joined forces with Sosei Heptares on a drug discovery project. The goal was to create new potential remedies targeting GPCR receptors in several therapeutic sectors. Many of these targets provide potential therapeutic intervention points, but overcoming the inherent technological obstacles has been challenging.
To conquer these barriers, Pfizer and Sosei Heptares researchers paired their unique skills in GPCR-focused, structure-based drug development directed at the GPCR targets picked by Pfizer. Pfizer assumes responsibility for the development and promotion of potential therapeutic agents for each selected target, holding exclusive worldwide rights to any possible treatment outcomes.
Until now, Sosei Heptares has presented several stabilized receptors, X-ray structures, and biophysical data on certain projects. Pfizer has chosen three distinct clinical candidates from their collaboration, which are now progressing into and through the initial clinical trial phase. This partnership has triggered numerous milestone payments so far from Pfizer, and further payments and potential royalties are anticipated under the agreement.
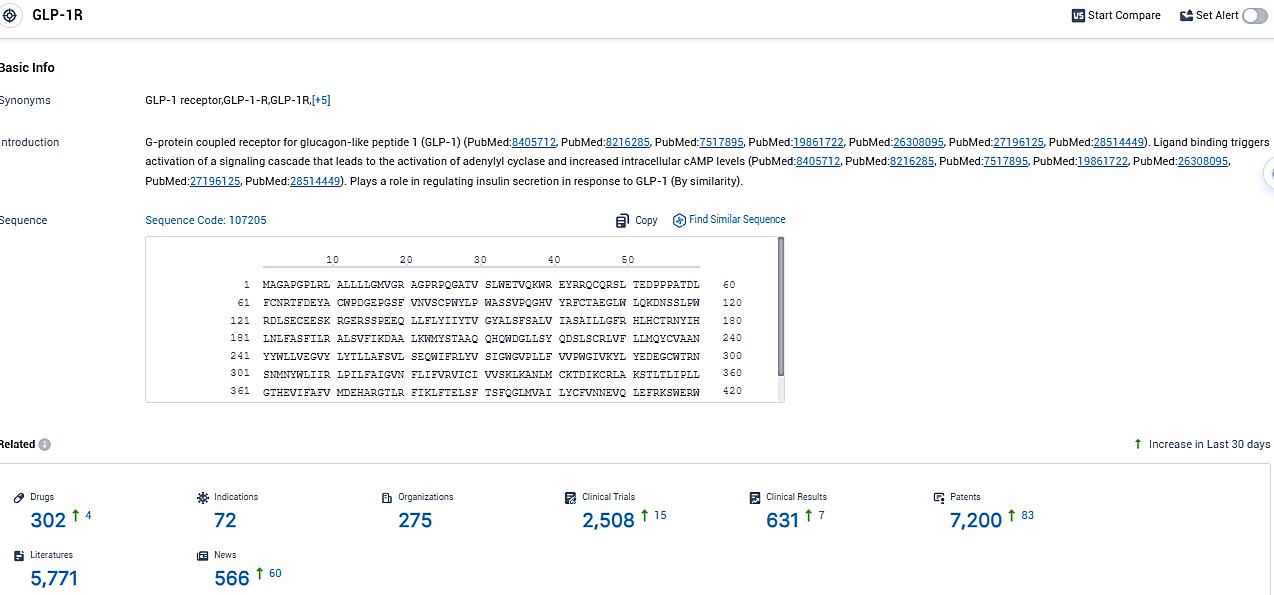
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 12, 2023, there are 302 investigational drugs for the GLP-1R target, including 72 indications, 275 R&D institutions involved, with related clinical trials reaching 2508, and as many as 7200 patents.
PF-06954522 has reached Phase 1 of clinical development, indicating progress in its evaluation for safety and dosage. As a potential therapeutic option in the field of biomedicine, further research and development will be required to determine its efficacy and potential impact on.