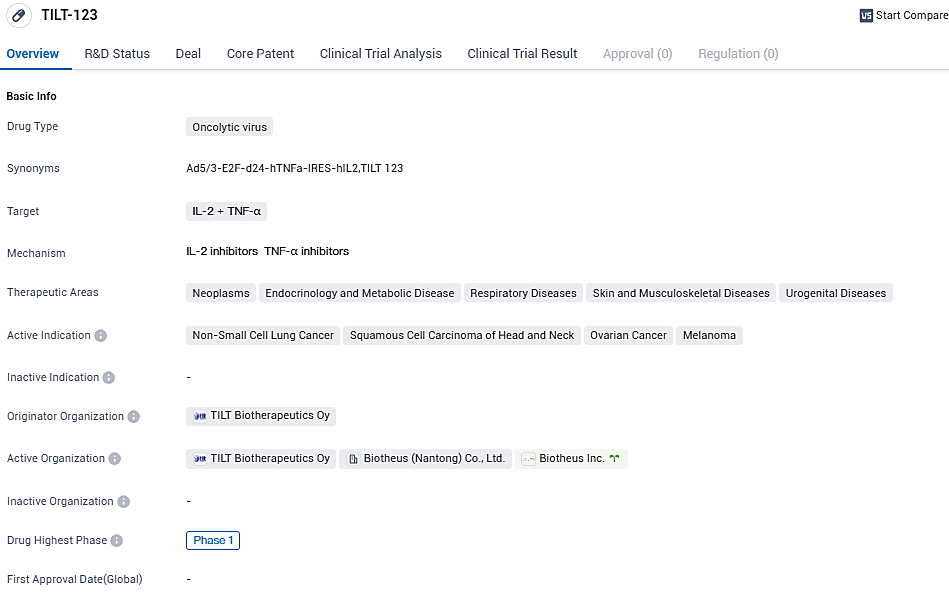
TILT Biotherapeutics reports encouraging clinical results for primary resource TILT-123 at 2023 Cancer Immunotherapy Society
At the Society for Immunotherapy of Cancer 2023 meeting, TILT Biotherapeutics, a pioneering clinical-grade biotech firm working on cancer immunotherapies, unveiled the first phase results for its TILT-123 solo treatment. This treatment, an oncolytic virus which activates T-cells, is targeted at patients with progressive solid tumors.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Importantly, TILT-123 monotherapy displayed great tolerability, aligning with other oncolytic virus treatments, which paves the way for possible combination therapies. There are currently a number of these trials in progress, demonstrating TILT's dedication to advancing cancer therapy methods.
Akseli Hemminki, the founder and CEO of TILT Biotherapeutics, is a cancer clinician who has directly treated a significant number of cancer patients using oncolytic viruses. He stated, “Our aim is to progress cancer immunotherapy and we will persistently look for combined therapies and innovative therapeutic strategies. We express our gratitude to the patients, as well as all our academic, clinical, and industrial partners for their indispensable involvement in the research."
TILT-123 is an oncolytic virus that has been programmed to code for interleukin-2 and tumor necrosis factor alpha. This helps in attracting, multiplying, and activating T-cells to rejuvenate the cancer microenvironment. TILT's technique involves oncolytic viruses that specifically replicate within and break down the cancer cells, simultaneously triggering immune reactions against the tumor.
The evaluation of the safety of TILT-123 monotherapy in patients with highly progressed tumors that are resistant to standard therapy is the purpose of the TUNIMO study, a stand-alone open-label phase 1 clinical trial. 20 patients participated, and the most common cancer types were sarcomas (35%), melanoma (15%), and ovarian cancer (10%).
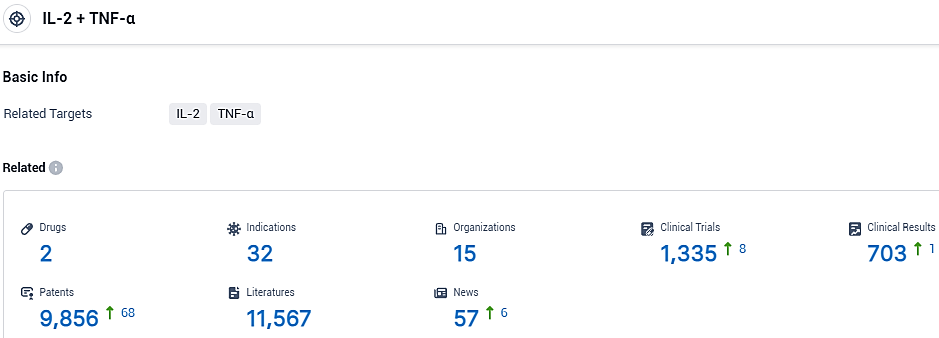
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 12, 2023, there are 2 investigational drugs for the IL-2 and TNF-α target, including 32 indications, 15 R&D institutions involved, with related clinical trials reaching 1335, and as many as 9856 patents.
TILT-123 shows promise as a potential treatment for various types of cancer. Its current phase of development suggests that it is still in the early stages of clinical testing. Further research and clinical trials will be needed to determine its safety and efficacy. If successful, TILT-123 could offer new options for patients with non-small cell lung cancer, squamous cell carcinoma of the head and neck, ovarian cancer, and melanoma, potentially improving their prognosis and quality of life.