Pharmaceutical Insights: Dydrogesterone's R&D Progress
Dydrogesterone's R&D Progress
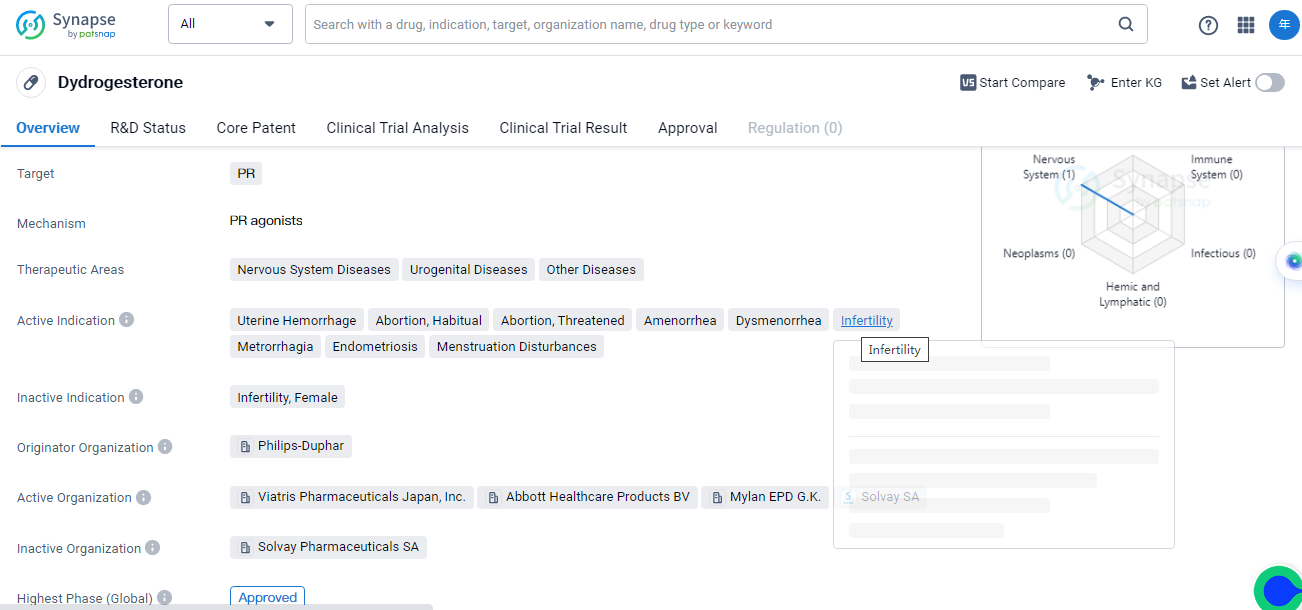
Dydrogesterone is a small molecule drug that primarily targets the progesterone receptor (PR). It is used in the treatment of various conditions related to the nervous system diseases, urogenital diseases, and other diseases. The drug has been approved for use in multiple therapeutic areas, including uterine hemorrhage, abortion (including habitual abortion and threatened abortion), amenorrhea, dysmenorrhea, infertility, metrorrhagia, endometriosis, and menstruation disturbances.
Dydrogesterone was first approved for use in the United Kingdom in January 1961. It is important to note that the drug's originator organization is Philips-Duphar. This suggests that Philips-Duphar was responsible for the initial development and commercialization of dydrogesterone.In terms of its development, dydrogesterone has reached the highest phase of approval globally.
Dydrogesterone's approval in multiple therapeutic areas highlights its versatility and potential to address a range of medical conditions. Its ability to target the progesterone receptor makes it particularly useful in the treatment of disorders related to the female reproductive system.
Given the objective nature of the provided information, it is important to avoid adding excessive subjective interpretations. However, based on the data provided, Dydrogesterone appears to be a well-established drug with a long history of use. Its approval in multiple countries and therapeutic areas suggests that it has demonstrated clinical effectiveness and safety.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Dydrogesterone: PR agonists
PR agonists are a type of medication that act as agonists, or activators, of the progesterone receptor (PR). The progesterone receptor is a protein found in cells that binds to the hormone progesterone, which plays a crucial role in the female reproductive system and pregnancy. PR agonists mimic the effects of progesterone by binding to the receptor and activating it, leading to various physiological responses.
From a biomedical perspective, PR agonists are commonly used in the field of reproductive medicine. They can be prescribed to women who have hormonal imbalances or conditions such as irregular menstrual cycles, endometriosis, or infertility. By activating the progesterone receptor, PR agonists help regulate the menstrual cycle, promote the development and maintenance of the uterine lining, and support successful implantation and pregnancy.
Additionally, PR agonists may also be used in the treatment of certain types of cancer, such as breast cancer or endometrial cancer. In these cases, the goal is to inhibit the growth of cancer cells that are dependent on progesterone signaling. By using PR agonists, the receptor becomes occupied and prevents progesterone from binding, thereby reducing the growth-promoting effects of progesterone on cancer cells.
It is important to note that the specific usage and dosage of PR agonists may vary depending on the individual's condition and the medical judgment of healthcare professionals.
Drug Target R&D Trends for Dydrogesterone
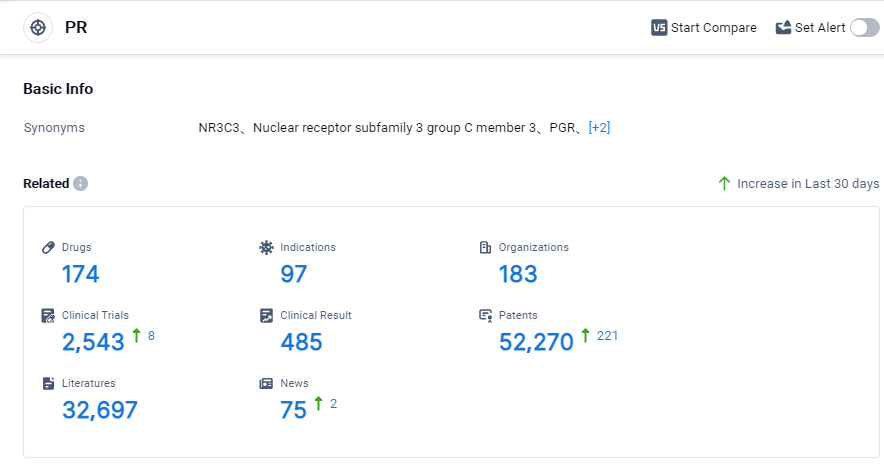
According to Patsnap Synapse, as of 3 Sep 2023, there are a total of 174 PR drugs worldwide, from 183 organizations, covering 97 indications, and conducting 2543 clinical trials.
Based on the analysis, it can be concluded that the target PR has a competitive landscape with multiple companies making progress in R&D. The future development of the target PR will likely see continued growth in the pharmaceutical industry, especially in countries like China.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In conclusion, Dydrogesterone is a small molecule drug that targets the progesterone receptor. It has been approved for use in various therapeutic areas, including nervous system diseases, urogenital diseases, and other diseases. Its approval dates back to 1961 in the United Kingdom, and it is currently approved in both global and Chinese markets. Dydrogesterone's versatility and efficacy make it a valuable option for the treatment of conditions such as uterine hemorrhage, abortion, amenorrhea, and endometriosis.