Pharmanovia Licenses Catumaxomab from Lindis Biotech for Malignant Ascites
Pharmanovia, an international pharmaceutical firm focused on the commercialization of innovative therapeutics and the enhancement, prolongation, and expansion of the lifecycle of existing drugs, has announced the growth of its oncology portfolio through a new licensing deal for catumaxomab, aimed at treating malignant ascites.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
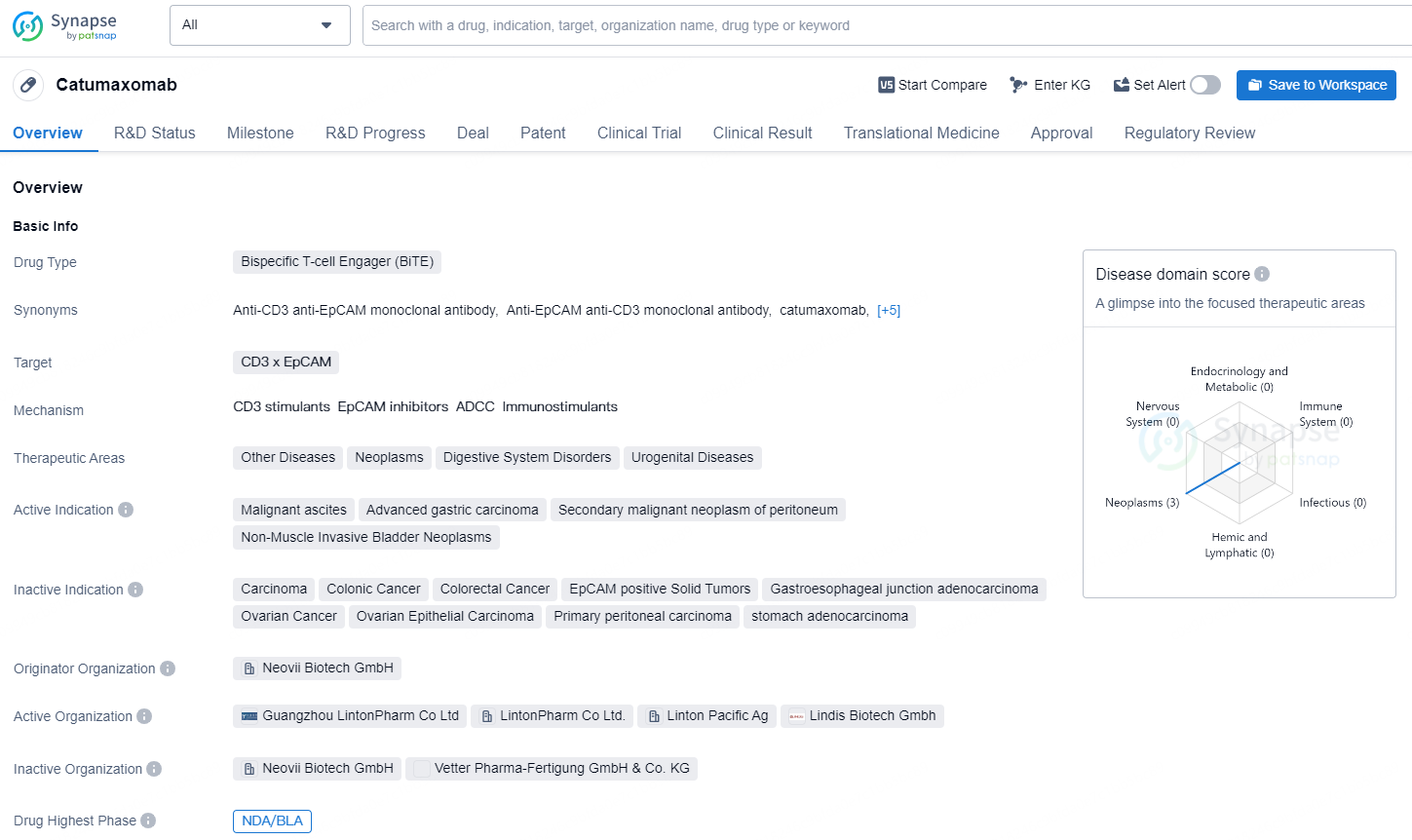
The agreement grants Pharmanovia the exclusive opportunity to commercialize catumaxomab, a pioneering trifunctional bi-specific monoclonal antibody designed for intraperitoneal therapy of malignant ascites in adult patients with epithelial cellular adhesion molecule (EpCAM)-positive carcinomas who are not suitable for additional systemic anticancer treatment. Malignant ascites refers to the abnormal build-up of fluid within the peritoneal cavity, often linked to advanced cancer stages.
Dr. Stephen Deacon, Chief Scientific Officer, notes: “The innovative aspect of catumaxomab lies in its targeted mechanism. This bispecific antibody (anti-EpCAM x anti-CD3) merges features of traditional monoclonal antibodies with bispecific formats. It attaches to tumor cells that express EpCAM, consequently boosting the activation of the patient's immune system to facilitate the destruction of tumor cells.”
Malignant ascites is a relatively uncommon condition, primarily associated with ovarian, pancreatic, and gastric cancers, occurring in 20 to 50% of all cases.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
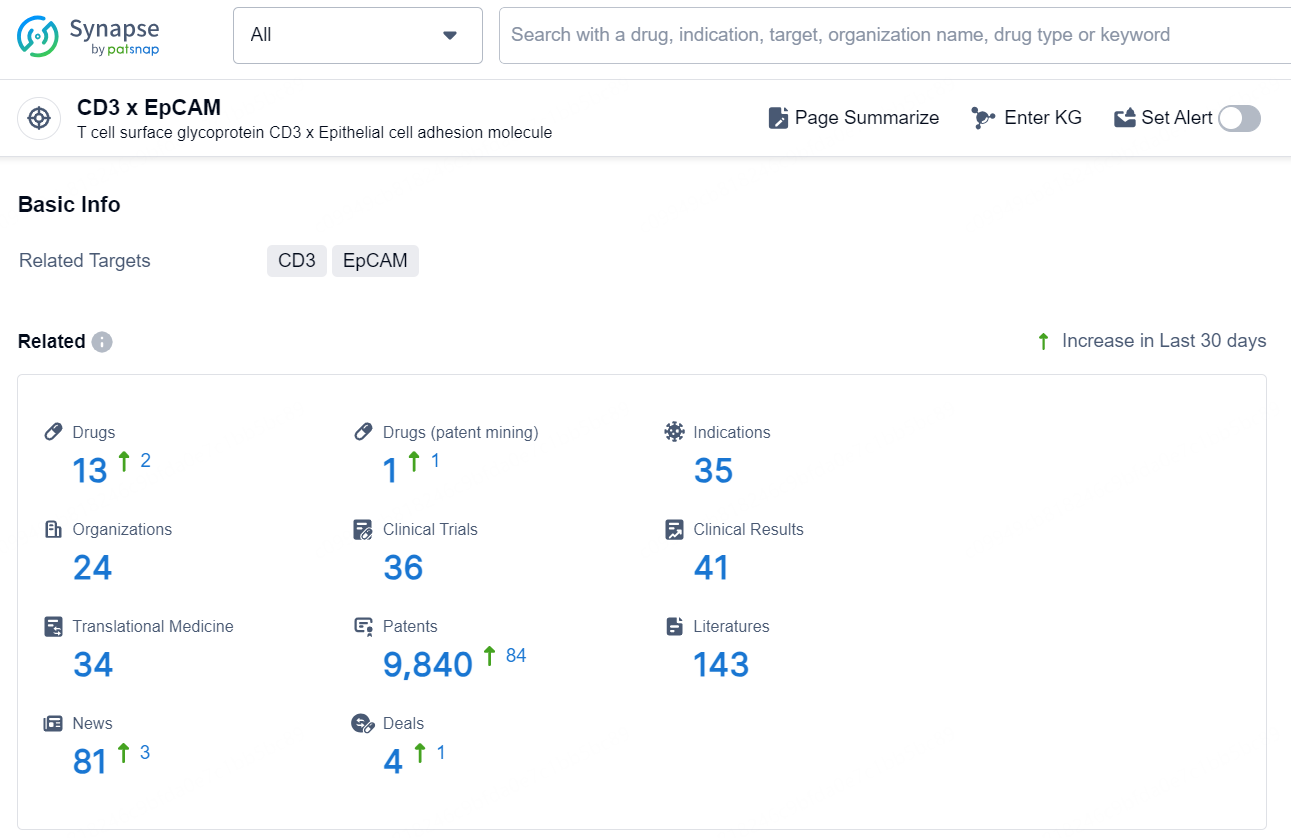
According to the data provided by the Synapse Database, As of November 25, 2024, there are 13 investigational drugs for the CD3 x EpCAM target, including 35 indications, 24 R&D institutions involved, with related clinical trials reaching 36, and as many as 9840 patents.
Catumaxomab is a bispecific T-cell engager (BiTE) drug that targets CD3 x EpCAM. It is used in a variety of therapeutic areas, including other diseases, neoplasms, digestive system disorders, and urogenital diseases.