Update on Transgene's Phase II Trial of TG4001 Cancer Vaccine for HPV16-Positive Cervical and Anogenital Cancers
Transgene (Euronext Paris: TNG), a biotechnology firm specializing in the creation and development of virus-based immunotherapies for cancer treatment, has reported that its Phase II randomized trial assessing TG4001 combined with avelumab compared to avelumab alone in individuals with recurrent or metastatic HPV16-positive cervical and anogenital cancers did not achieve the study's primary goal of enhancing progression-free survival.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
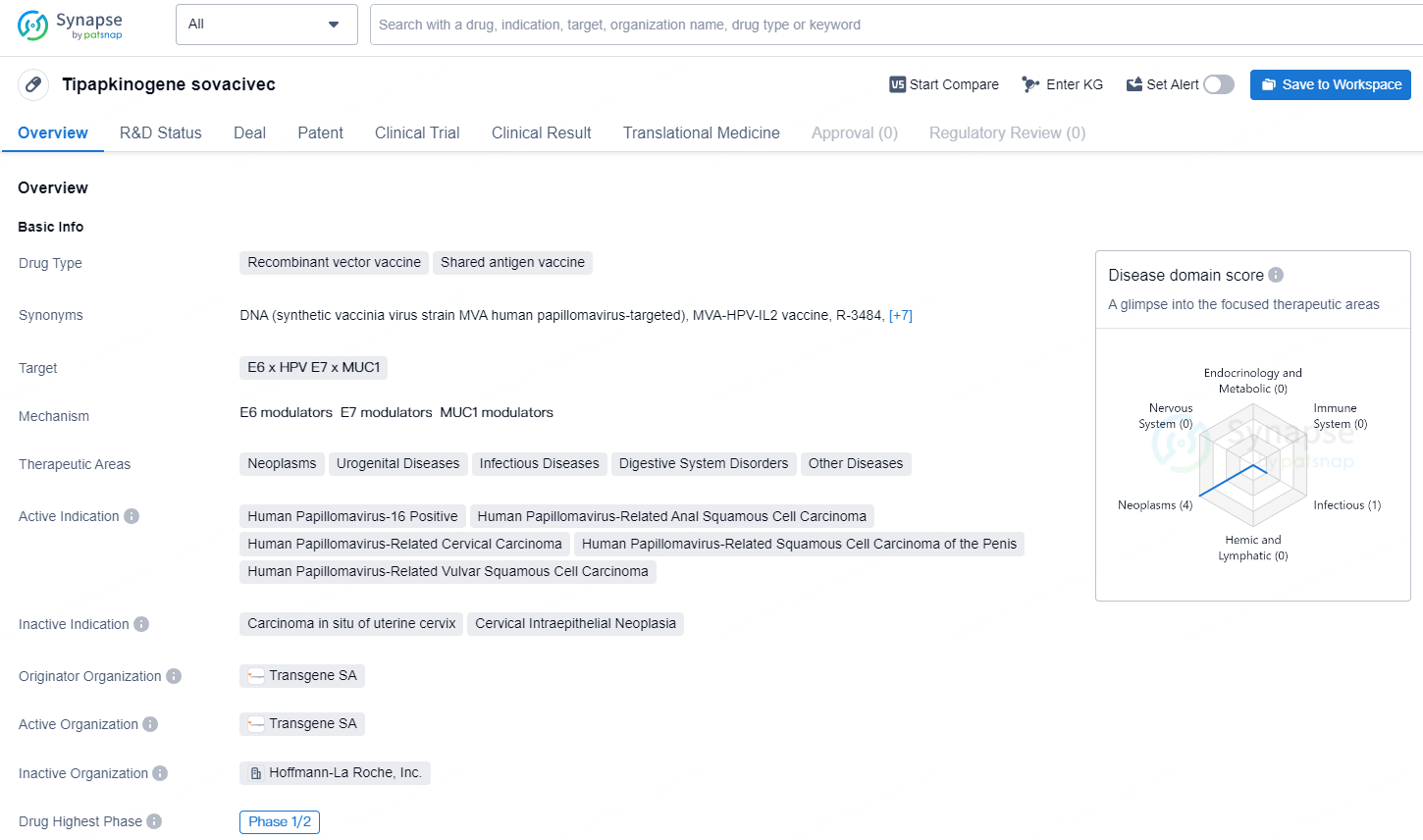
TG4001 (tipapkinogen sovacivec) is an experimental vaccine therapy utilizing a non-replicative, extensively weakened Vaccinia vector (MVA). This vector is customized to produce HPV16 antigens (E6 & E7) and an immune-enhancing agent (IL-2). TG4001 aims to engage in a dual antiviral strategy: first, by training the immune system to specifically identify cells that display the HPV16 E6 and E7 antigens present in HPV16-linked malignancies, and second, by enhancing the immune system's capability to eliminate infections using interleukin 2 (IL-2). Over 350 individuals have been treated with TG4001. Its method of action and notable safety record suggest TG4001 is a strong candidate for use alongside other treatments for HPV-associated solid tumors.
A predetermined subgroup evaluation revealed a promising efficacy inclination favoring the regimen including TG4001 for cervical cancer patients. However, further validation through additional analyses, including assessments by PD-L1 status, is necessary. These individuals represent nearly half of the study's participants. The treatment has been well-received, with side effects aligning with earlier findings.
Transgene is currently meticulously reviewing the complete study outcomes to ascertain the most suitable path for this program and plans to provide additional updates once this review is completed.
Dr. Alessandro Riva, the Chairman and CEO of Transgene, expressed: “While not achieving the primary endpoint in our Phase II study with TG4001 is disappointing, the observed positive efficacy trend in favor of the combination regimen in cervical cancer patients is encouraging. We are committed to executing an exhaustive and thorough data analysis before plotting a future course for this asset, especially in cervical cancer, in light of the dynamic treatment environment. The comprehensive study results are expected to be disclosed at an upcoming scientific conference. We extend our gratitude to all patients and caregivers involved in this research for their critical contributions. Our strategic direction remains on progressing our flagship asset, TG4050, a personalized cancer vaccine for head and neck cancers to be utilized following surgery and adjuvant treatment. We anticipate sharing further data on TG4050 from the median 24-month follow-up of Phase I patients in our head and neck cancer trial in November 2024 during the SITC conference.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
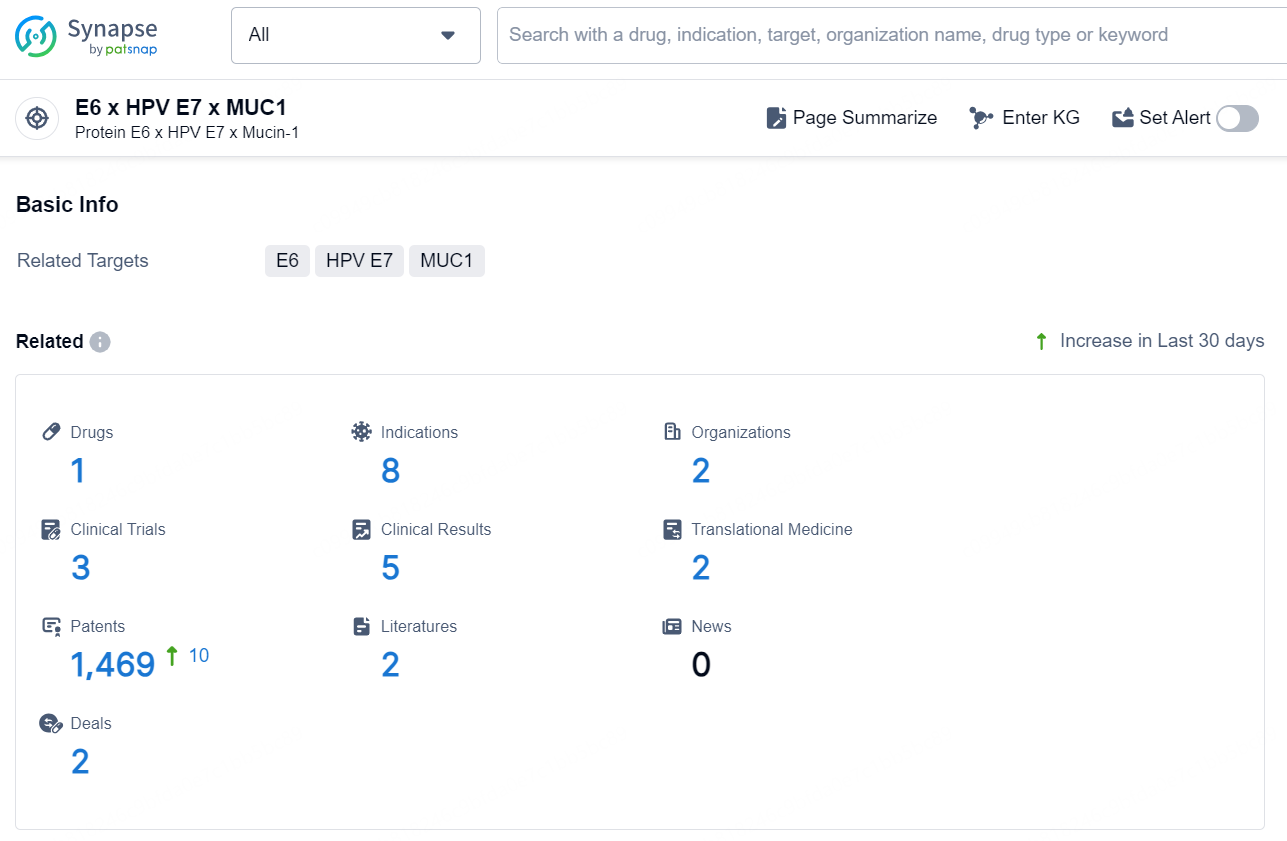
According to the data provided by the Synapse Chemical, As of October 16, 2024, there are 1 investigational drug for the E6 x HPV E7 x MUC1 target, including 8 indications, 2 R&D institutions involved, with related clinical trials reaching 3, and as many as 1469 patents.
Tipapkinogene sovacivec is a recombinant vector vaccine that falls under the category of shared antigen vaccines. It targets the E6 x HPV E7 x MUC1 and is designed to tackle a range of therapeutic areas including neoplasms, urogenital diseases, infectious diseases, digestive system disorders, and other diseases. The drug's active indication focuses on diseases related to the human papillomavirus, including Human Papillomavirus-16 Positive, Human Papillomavirus-Related Anal Squamous Cell Carcinoma, Human Papillomavirus-Related Cervical Carcinoma, Human Papillomavirus-Related Squamous Cell Carcinoma of the Penis, and Human Papillomavirus-Related Vulvar Squamous Cell Carcinoma.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!